Abstract
Although twins have lower birthweights than singletons, they may not experience the increased disease risk in adulthood reportedly associated with low birthweight. In contrast, another periconceptional event, maternal undernutrition, does not reduce birthweight but does affect fetal and postnatal physiology in sheep. We therefore studied maternal and fetal metabolism, growth and glucose–insulin axis function in late gestation in twin and singleton sheep pregnancies, either undernourished from 60 days before until 30 days after conception or fed ad libitum. We found that twin-bearing ewes had decreased maternal food intake in late gestation and lower maternal and fetal plasma glucose and insulin levels. Twin fetuses had fewer everted placentomes, grew slower in late gestation, and had a greater insulin response to a glucose challenge, but lesser response to arginine. In contrast, periconceptional undernutrition led to increased maternal food intake and a more rapid fall in maternal glucose levels in response to fasting. Periconceptional undernutrition increased the number of everted placentomes, and abolished the difference in insulin responses to glucose between twins and singletons. Thus, the physiology of twin pregnancy is quite different from that of singleton pregnancy, and is probably determined by a combination of factors acting in both early and late gestation. The inconsistency of the relationships between low birthweight and postnatal disease risk of twins may lie in their very different fetal development. These data suggest that twin pregnancy may be another paradigm of developmental programming, and indicate that twins and singletons must be examined separately in any study of fetal or postnatal physiology.
Both human and animal studies have demonstrated an association between reduced size at birth and altered postnatal physiology (Lithell et al. 1996; Oliver et al. 2002). Most of the experimental studies have involved multitocous species, or mixtures of twin and singleton pregnancies, without careful examination of any possible differences between these.
Twins commonly have reduced size at birth compared with singletons. However, the relationship between size at birth and postnatal physiology and disease risk is much less clear, with some studies of human twins reporting that reduced birthweight is associated with reduced glucose tolerance (Poulsen et al. 1997) or greater risk of type 2 diabetes (Iliadou et al. 2004), greater increases in blood pressure in infancy (Levine et al. 1994), increased risk of hypertension in adulthood (Bergvall et al. 2007) and increased hypothalamic–pituitary–adrenal axis responsiveness (Wust et al. 2005), whereas others report no increase in blood pressure, either in childhood (Zhang et al. 2001) or adulthood (Baird et al. 2001), or a reduction in glucose tolerance in adulthood (Baird et al. 2001) with decreasing birthweight.
There are many possible reasons for this lack of clarity including small sample sizes and use of selected populations (Phillips et al. 2001). Many studies also compare associations between birthweight and outcomes within twin pairs or between monozygotic and dizygotic twin pairs. If, however, the fact of being a twin is itself responsible for both reduced size at birth and increased disease risk, then all twins will be affected, and analyses that compare differences in birth size between monozygotic and dizygotic twin pairs will have reduced power to detect an association unless coefficients for both the between-twin pair and the within-twin pair effect of birthweight on the outcome of interest are included (Carlin et al. 2005; Poulsen & Vaag, 2006). The between-twin pair coefficient takes account of factors affecting birthweight that may affect both twins equally, for example, maternal factors such as nutritional status or body habitus. The within-twin pair coefficient takes account of factors affecting birthweight that are specific to each fetus, which may include both genetic and environmental factors specific to each fetus (such as the metabolic and endocrine milieu, or the transplacental nutrient supply that each fetus receives). The finding in both human and animal studies that the associations between birthweight and postnatal physiology are stronger for the within-twin pair than the between-twin pair association (Dwyer et al. 1999; Bloomfield et al. 2007) suggest that individual environmental factors affecting fetal growth in twins are more important than genetic or maternal factors.
It is also possible that the physiology of twin pregnancy is fundamentally different from that of singleton pregnancy, so that both the causes of low birthweight and the mechanisms underlying the associations with postnatal disease risk are also different. Studying differences between twin and singleton pregnancies in fetal growth patterns and physiology may help explain why these associations are less clear for twins, and may suggest mechanisms underlying these relationships.
Twinning is a periconceptional event, and twins develop in a different environment to singletons from conception at least until weaning. Throughout gestation twin fetuses compete for maternal nutrients, have smaller placentae than singletons, and restricted physical space. After delivery twins compete for milk supply and parental attention. Maternal metabolic and nutritional status before conception can influence the likelihood of a twin conception (Kenyon et al. 2004), but differences in maternal metabolic status later in pregnancy between twin and singleton pregnancies have received little attention.
Reduced birth size and ponderal index in singletons, which correlates with the risk of adult metabolic disease, is most often due to growth limitation in late gestation. In twins, a reduced fetal growth trajectory may be present from early in gestation (Iffy et al. 1983; Xu et al. 1995). However, the finding that human triplets reduced to twins at 8–11 weeks are heavier at birth than the non-reduced triplets (Boulot et al. 2000), but not as heavy as non-reduced twins (Sebire et al. 1997) suggests both early and late gestation effects.
We have previously reported the effects of another periconceptional influence, that of maternal undernutrition, on fetal growth and physiology in sheep with singleton pregnancies. Mild undernutrition of the ewe before and for the first month of pregnancy resulted in an altered maternal metabolic and endocrine environment throughout pregnancy (Oliver et al. 2005). Fetal growth trajectory was slowed with altered growth responses to a nutritional challenge in late gestation (Harding, 1997b) but size at birth was unaffected (Oliver et al. 2005) when corrected for earlier birth in the fetuses of undernourished ewes (Bloomfield et al. 2003b). Fetal metabolic and endocrine maturation was also perturbed (Bloomfield et al. 2004), including that of the glucose–insulin axis (Oliver et al. 2001). At least some of these effects persist after birth (Todd et al. 2007).
Thus, both twinning and periconceptional undernutrition result in altered fetal growth in late gestation, and are initiated at a similar time in pregnancy. If the physiology of twin pregnancy is primarily determined in the periconceptional period, a twin pregnancy may have similar features in late gestation to those of periconceptional undernutrition. We hypothesized that, when compared with well-nourished singleton pregnancy, both twinning and periconceptional undernutrition would result in similar alterations in maternal metabolism in late gestation, fetal growth trajectory in response to a late gestation nutritional insult, and maturation of the fetal glucose–insulin axis. Conversely, if the physiology of twin pregnancy is also determined by additional influences in late gestation, there may be an interaction between the effects of twinning and those of periconceptional undernutrition. We therefore compared maternal metabolism, fetal growth and glucose–insulin axis function between singleton and twin pregnancies in ewes that were either well-nourished or exposed to periconceptional undernutrition.
Methods
Animals
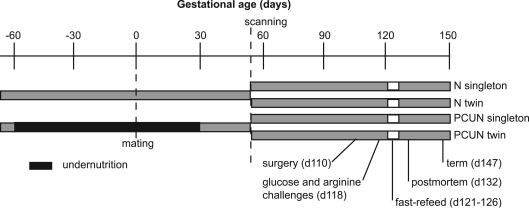
Ethical approval for the study was obtained from the University of Auckland Animal Ethics Committee. After acclimatization to a concentrate feed (CamTech, Cambridge, New Zealand (Oliver et al. 2005)), non-pregnant Romney ewes were randomly allocated to maintenance feeds (N; concentrate feeds at 3–4% of body weight per day), or periconceptional undernutrition from 60 days before until 30 days after mating (PCUN; 2 days fast, then individually adjusted concentrate feeds to achieve and maintain a 10–15% weight loss). Initially, feed intake in the PCUN group was 1–2% of body weight per day, but by mating was 80% of intake in N ewes. Following the period of undernutrition all ewes were fed maintenance feeds. A fortnight before mating with Dorset rams, the oestrous cycle of all ewes was synchronized (Wheaton et al. 1993). Singleton and twin pregnancies were established by ultrasound scanning at 55 days, giving four experimental groups: N ewes carrying singleton fetuses; N ewes carrying twin fetuses; PCUN ewes carrying singleton fetuses, and PCUN ewes carrying twin fetuses (Fig. 1). Maternal blood samples were taken by jugular puncture at regular intervals from 71 days before mating until transport to the laboratory at 104 days, and ewes were weighed at least twice weekly.
Figure 1. Timeline of experimental protocol.
PCUN, periconceptional undernutrition; N, well-nourished throughout.
After acclimatization to the laboratory for 5 days, the ewes underwent surgery under general anaesthesia (induction with Alfaxan-CD RTU, 0.35 ml kg−1, Jurox Pty Ltd, Rutherford, NSW, Australia; maintainenance with 2% halothane and oxygen) at 111 ± 1 days gestation for placement of maternal and fetal arterial, venous and amniotic catheters. Fetal chest girth and hindlimb length were measured and growth catheters were fitted around the chest of the fetus (Harding, 1997a). Both fetuses were catheterized in the case of twins.
Baseline maternal and fetal blood samples were taken in the morning before feeding on days 114, 117, 121, 127 and 131. Samples were placed on ice, then centrifuged at 2500 g for 10 min at 4°C and plasma stored at −80°C.
On day 118, after an overnight fast, a glucose challenge was performed on the fetuses. After a baseline blood sample, 1.5 g glucose (3 ml 50% dextrose) was given intravenously to the fetus, and arterial blood samples were taken at 2, 5, 10, 15, 30, 45 and 60 min. Four hours later, when glucose and insulin had returned to baseline, an arginine challenge was performed. After a baseline blood sample, 300 mg arginine (100 mg ml−1 in normal saline, Sigma Chemical Co., St Louis, MO, USA) was given intravenously to the fetus and arterial blood samples were taken at 2, 5, 10, 15, 30, 45 and 60 min. Challenges were performed simultaneously in twin fetuses to minimize any clearance of plasma glucose and arginine from one twin to another via any placental vascular anastamoses.
Ewes were fasted from day 121–124, and maternal and fetal blood samples were taken daily. On day 124 ewes were refed and also given an intravenous glucose infusion of 25 g over 8 h, aiming to restore maternal and hence fetal blood glucose as rapidly as possible. Further blood samples were collected 2, 4, 6 and 8 h after the start of the glucose infusion, and at 24 and 48 h of refeeding.
The sheep were killed with an overdose of pentobarbitone on day 132 and a postmortem performed. Twin fetuses were designated ‘heavy’ or ‘light’ according to whether they were the heavier or lighter of the pair at postmortem. The fetus and placenta were measured, dissected and weighed, and placentomes were categorized into four groups (A–D) according to morphology, where ‘A’ type placetomes are inverted with a preponderance of paler maternal tissue visible and ‘D’ type are everted with a preponderance of darker fetal tissue visible (Vatnick et al. 1991). ‘B’ and ‘C’ placentomes are intermediate. For twins, placentomes were assigned to the relevant twin, and placental mass is reported per twin rather than total placental mass for the pregnancy.
Hormone and metabolite assays
Metabolite concentrations were measured on an Hitachi 902 autoanalyser (Hitachi, Tokyo, Japan) using commercial kits (glucose and urea kits from Roche, Mannheim, Germany; lactate, free fatty acids (FFA) and β-hydroxy butyrate (βHBA) kits from Randox Laboratories Ltd, Ardmore, Crumlin, UK). Plasma insulin was measured by radioimmunoassay (RIA) (Oliver et al. 1993) with ovine insulin as the standard (Sigma, batch no. I9254). The minimal detectable concentration was 0.03 ng ml−1; inter- and intra-assay coefficients of variation (CVs) were 11.1% and 11.0%, respectively. Plasma IGF-1 was measured using an IGFBP-blocked RIA (Blum & Breier, 1994). The detection limit was 0.7 ng ml−1; inter- and intra-assay CVs were 17.5% and 10.0%, respectively.
Fetal growth
Fetal growth catheters were measured daily. Fetal weight was estimated as follows: firstly, chest girth was calculated at a particular gestational age by subtracting the measured growth catheter increment from measured chest girth at postmortem. Secondly, the ratio of fetal weight to chest girth was calculated from measurements at postmortem and then estimated at a particular gestational age by extrapolating backwards at a rate of 0.2 g mm−1 day−1. This figure was derived from a large data pool (n = 116) of fetal postmortems, in which the slope of a least squares line through the fetal weight to chest girth ratios versus gestational age was 0.20 ± 0.02 g mm−1 day−1 (r2= 0.4, P < 0.0001), and was not different between twins and singletons. Fetal weight at a particular gestational age was then calculated by multiplying the estimated chest girth by the estimated fetal weight to chest girth ratio.
Analysis of growth data from the pre-fasting period used data from 114 to 121 days, the fasting period used data from 122 to 124 days, and post-fasting from 125 to 131 days.
Statistics
Statistical analyses were performed using JMP 5.1 (SAS Institute Inc., Cary, NC, USA). Maternal food intake was compared between 114 and 131 days, excluding days on which sheep were fasted for experiments. Metabolite and hormone data were averaged over the periconceptional period (day −60 to 30), mid gestation (day 40 to 97), and late gestation when in the laboratory (day 114 to 121) for display in tables.
Maternal metabolite and hormone data were compared using a two-way ANOVA with singleton/twin status, nutritional group, and the interaction between these terms as independent variables, and the Tukey–Kramer correction for multiple comparisons. A similar two-way repeated measures ANOVA was used for comparing the fasting and refeeding periods. Maternal to fetal glucose gradients were calculated as the difference between maternal and fetal plasma glucose levels, using data from all samples excluding the challenges and fasting-refeeding period.
For the fetal glucose and arginine challenges, area under the curve (AUC) was calculated from baseline. Fetal data were compared using a two-way ANOVA with singleton/twin status, nutritional group, and their interaction as independent variables, and with ewe number nested within nutritional group and singleton/twin status to identify twin pairs as arising from the same ewe, thereby allowing for the non-independence of twins. The Tukey–Kramer method was used to correct for multiple comparisons. Estimated fetal weight was also included as a covariate in the analyses of the glucose and arginine challenge data to account for the variable dose of glucose per kilogram of fetal body weight.
Fetal growth was compared over pre-fasting, fasting and post-fasting periods using multiple linear regression with singleton/twin status, nutritional group, and their interaction as independent variables, and with ewe number nested within nutritional group and singleton/twin status as above. These analyses included variables identifying the preceding period (pre-fasting or fasting, respectively) and the temporal relationship of measurements so that growth rate was not treated independently of prior growth rate (Harding, 1997a).
Metabolite and hormone concentrations were compared using a two-way ANOVA with heavy/light, nutritional group, and their interaction as independent variables.
Data are presented as mean ± standard error of mean (s.e.m.).
Results
Twenty-eight singleton-bearing ewes (15 N, 13 PCUN) entered the experiment, 22 (10 N, 12 PCUN) completed the fast-refeed protocol, and postmortem data were available for 19 (9 N, 10 PCUN). Twenty-eight twin-bearing ewes (13 N, 15 PCUN) entered the experiment, 22 (12 N, 10 PCUN) completed the fast-refeed protocol, and postmortem data were available for 19 (11 N, 8 PCUN). Not all samples were available from all animals, due to catheter failures and some fetal losses.
The average weight loss due to periconceptional undernutrition was 14.8 ± 1.2% in singleton and 16.3 ± 2.5% in twin-bearing ewes. There was no weight difference between singleton and twin-bearing ewes at day 110, but PCUN ewes were still lighter than N ewes (Table 1).
Table 1.
Maternal weight (kg)
| Singleton-bearing ewes | Twin-bearing ewes | |||
|---|---|---|---|---|
| N (n = 15) | PCUN (n = 13) | N (n = 13) | PCUN (n = 15) | |
| Day −71 | 64.1 ± 1.3 | 65.0 ± 1.5 | 62.9 ± 1.9 | 66.0 ± 1.5 |
| Day −2 | 65.9 ± 1.5 | 55.2 ± 1.3** | 64.1 ± 1.8 | 55.6 ± 1.1** |
| Day 110 | 68.5 ± 1.5 | 62.5 ± 1.6* | 67.5 ± 1.1 | 66.8 ± 1.6* |
Data are mean ±s.e.m. PCUN, periconceptional undernutrition. See text for detail of nutritional groups.
P < 0.01
P < 0.05 for nutrition effect.
Fetal growth
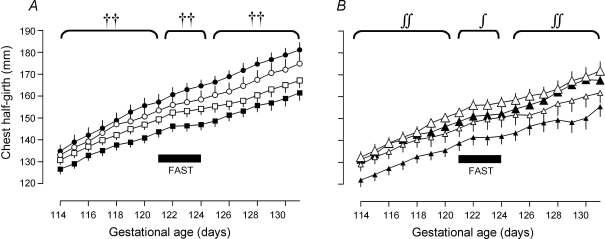
At surgery, twin fetuses had smaller chest girths than singletons (Table 2). They then grew more slowly than singletons from 114 to 121 days (average girth increment 2.51 ± 0.07 versus 2.95 ± 0.12 mm day−1, P < 0.01), and slowed their growth by 76% with maternal fasting (to 0.59 ± 0.19 mm day−1) compared with 46% for singletons (to 1.58 ± 0.31 mm day−1) (P < 0.01 for single/twin by time effect) (Fig. 2). Chest girth increment increased with refeeding, but was still less in twin fetuses than in singletons (2.05 ± 0.10 versus 2.48 ± 0.14 mm day−1, P < 0.01).
Table 2.
Fetal morphometric data at day 111 and 132
| Singletons | Twins | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | PCUN (n = 11) | N (n = 22) | PCUN (n = 18) | ||||||
| Gestational | Morphometric | (n = 9) | (n = 11) | ||||||
| age | measurement | Overall | Heavy | Light | Overall | Heavy | Light | ||
| Day 111 | Chest girth (cm) | 27.0 ± 0.5 | 26.2 ± 0.8 | 25.3 ± 0.4† | 26.3 ± 0.5§ | 24.4 ± 0.6 | 26.3 ± 0.3† | 26.5 ± 0.4§ | 26.0 ± 0.5 |
| Hindlimb length (cm) | 25.9 ± 0.5 | 25.5 ± 0.3 | 25.2 ± 0.3 | 25.2 ± 0.4 | 25.2 ± 0.4 | 25.6 ± 0.2 | 25.6 ± 0.4 | 25.6 ± 0.3 | |
| Day 132 | Weight (g) | 4393 ± 166 | 4317 ± 128 | 3614 ± 144†† | 3883 ± 207§§ | 3345 ± 166 | 3665 ± 86†† | 3844 ± 113§§ | 3487 ± 97 |
| Crown—rump length (cm) | 47.3 ± 1.0 | 46.4 ± 0.6 | 42.8 ± 0.8†† | 43.5 ± 1.3 | 42.1 ± 0.8 | 43.9 ± 1.1†† | 44.8 ± 0.9 | 43.1 ± 2.0 | |
| Chest girth (cm) | 34.6 ± 0.6 | 35.3 ± 0.7 | 32.0 ± 0.6†† | 32.8 ± 0.7 | 31.2 ± 0.8 | 32.5 ± 0.4†† | 32.5 ± 0.6 | 32.5 ± 0.6 | |
| Hindlimb length (cm) | 33.7 ± 0.5 | 33.3 ± 0.5 | 31.0 ± 0.4†† | 31.4 ± 0.7 | 30.5 ± 0.5 | 32.4 ± 0.4†† | 32.8 ± 0.7 | 32.1 ± 0.4 | |
| Placental weight (g) | 552 ± 28 | 593 ± 38 | 382 ± 23†† | 396 ± 324 | 368 ± 36 | 382 ± 18†† | 373 ± 28 | 391 ± 25 | |
| Number of placentomes | 81.6 ± 3.7 | 77.2 ± 6.6 | 52.3 ± 2.7†† | 54.4 ± 3.60 | 50.1 ± 4.1 | 58.5 ± 3.1†† | 53.9 ± 2.7 | 63.1 ± 5.4 | |
| % A placentomes | 22 ± 9 | 10 ± 3 | 21 ± 6 | 21 ± 8 | 25 ± 9 | 22 ± 6 | 25 ± 9 | 21 ± 10 | |
| % B placentomes | 44 ± 9 | 37 ± 9 | 48 ± 7 | 55 ± 8 | 43 ± 10 | 44 ± 7 | 43 ± 10 | 42 ± 10 | |
| % C placentomes | 18 ± 6 | 20 ± 5 | 21 ± 6 | 19 ± 6 | 11 ± 4 | 14 ± 4 | 11 ± 4 | 22 ± 10 | |
| % D placentomes | 15 ± 10 | 32 ± 11** | 9 ± 3† | 4 ± 3 | 21 ± 10 | 20 ± 7†** | 21 ± 10 | 15 ± 6 | |
Data are mean ±s.e.m. PCUN, periconceptional undernutrition. See text for detail of nutritional groups. Placental weights in twins refer to placental weight per twin.
P < 0.01 for nutrition effect
P < 0.01
P < 0.05 for twin effect
P < 0.01
P < 0.05 for heavy/light effect.
Figure 2. Fetal growth curves.
A, fetal growth curves in singleton and twins. •, singleton N; ○, singleton PCUN; ▪, twin N; □, twin PCUN. PCUN, periconceptional undernutrition. Symbols represent effect on growth rate. ††P < 0.01 for twin effect. B, fetal growth curves in twins divided into heavy and light twins. Large filled triangle, twin heavy N; small filled triangle, twin light N; large open triangle, twin heavy PCUN; small open triangle, twin light PCUN. PCUN, periconceptional undernutrition. Symbols represent effect on growth rate. ∫∫P < 0.01, ∫P < 0.05 for heavy/light effect. ∫P < 0.05 for heavy/light × nutrition interaction.
Twins had smaller chest girths, were significantly lighter and shorter and had shorter limbs than singletons at postmortem on day 132 (Table 2). Placentome number and placental weight per fetus were also lower in twins than singletons, with ‘D’ placentomes contributing a smaller proportion of placental weight in twins (Table 2). There was no effect of PCUN on size at surgery (Table 2) or growth rates (PCUN versus N: pre-fast 2.64 ± 0.10 versus 2.66 ± 0.08 mm day−1, P = 0.41; fast 1.02 ± 0.24 versus 0.82 ± 0.24 mm day−1, P = 0.41; post-fast 2.27 ± 0.14 versus 2.16 ± 0.10 mm day−1, P = 0.59). There was also no effect of PCUN on fetal or placental measurements at postmortem. However, ‘D’ placentomes contributed a greater proportion of placental weight in PCUN animals (Table 2).
Within twin pairs, the heavier fetus had a larger chest girth at surgery (Table 2), and a greater rate of increase in chest girth from 114 to 121 days (2.64 ± 0.11 versus 2.37 ± 0.09 mm day−1, P < 0.01). Although there was no difference in daily chest girth increment during the fast between heavy and light fetuses, there was an interaction between heavy/light and nutritional group such that the light fetus in N sheep grew more slowly during the fast than the light fetus in PCUN sheep (0.23 ± 0.31 versus 1.08 ± 0.57 mm day−1, P < 0.05). After refeeding, heavy fetuses continued to have a greater daily chest girth increment than light fetuses (2.38 ± 0.15 versus 1.69 ± 0.13 mm day−1, P < 0.01) (Fig. 2). There was no significant difference in other morphometric measurements or placental size at postmortem between heavy and light fetuses.
Metabolism
Maternal
Plasma metabolite and hormone concentrations were not different in twin and singleton-bearing N ewes in early and mid-gestation, but in late gestation twin-bearing ewes had lower plasma glucose and insulin, and higher plasma FFA and ketone concentrations than singleton-bearing ewes (Table 3). Maternal food intake from 114 days was 14% less in twin than singleton-bearing ewes (1802 ± 76 versus 2099 ± 68 g day−1, P < 0.01).
Table 3.
Maternal and fetal plasma metabolite and hormone levels
| Twins | ||||||
|---|---|---|---|---|---|---|
| Gestational age range | Singletons N (n = 10) | PCUN (n = 12) | N (n = 12) | PCUN (n = 10) | ||
| Glucose (mmol.l−1) | ||||||
| Maternal | −61 | 4.02 ± 0.14 | 4.08 ± 0.26 | 3.99 ± 0.14 | 3.80 ± 0.09 | |
| −60 to 30 | 3.71 ± 0.10 | 3.48 ± 0.07** | 3.78 ± 0.05 | 3.53 ± 0.06** | ||
| 40 to 97 | 3.74 ± 0.10 | 3.55 ± 0.07 | 3.65 ± 0.07 | 3.65 ± 0.05 | ||
| 114 to 121 | 3.69 ± 0.06 | 3.53 ± 0.05 | 3.33 ± 0.07†† | 3.31 ± 0.07†† | ||
| Fetal | 114 to 121 | 1.03 ± 0.04 | 0.91 ± 0.05* | 0.80 ± 0.02†† | 0.81 ± 0.03†† | |
| Insulin (ng.ml−1) | ||||||
| Maternal | −61 | 0.19 ± 0.03 | 0.19 ± 0.05 | 0.14 ± 0.03 | 0.15 ± 0.02 | |
| −60 to 30 | 0.18 ± 0.02 | 0.13 ± 0.01** | 0.18 ± 0.02 | 0.11 ± 0.02** | ||
| 40 to 97 | 0.20 ± 0.04 | 0.11 ± 0.02* | 0.19 ± 0.02 | 0.14 ± 0.03* | ||
| 114 to 121 | 0.33 ± 0.03 | 0.25 ± 0.02* | 0.17 ± 0.02†† | 0.16 ± 0.01*†† | ||
| Fetal | 114 to 121 | 0.20 ± 0.02 | 0.21 ± 0.02 | 0.16 ± 0.02†† | 0.14 ± 0.02†† | |
| IGF1 (ng.ml−1) | ||||||
| Maternal | −61 | 44.3 ± 3.6 | 41.7 ± 2.4 | 40.3 ± 3.6 | 34.7 ± 2.1 | |
| −60 to 30 | 44.2 ± 2.1 | 41.3 ± 2.0 | 43.1 ± 3.2 | 42.6 ± 1.8 | ||
| 40 to 97 | 46.5 ± 2.2 | 44.4 ± 2.0 | 47.6 ± 4.9 | 45.2 ± 1.5 | ||
| 114 to 121 | 77.6 ± 9.9 | 46.6 ± 5.0* | 70.1 ± 7.1 | 56.9 ± 8.0* | ||
| Fetal | 114 to 121 | 71.5 ± 6.8 | 62.4 ± 6.1 | 68.7 ± 3.5 | 74.2 ± 3.0 | |
| Beta hydroxy butyrate (mmol.l−1) | ||||||
| Maternal | −61 | 0.15 ± 0.01 | 0.18 ± 0.02 | 0.16 ± 0.02 | 0.19 ± 0.02 | |
| −60 to 30 | 0.19 ± 0.01 | 0.21 ± 0.01** | 0.17 ± 0.01 | 0.25 ± 0.01** | ||
| 40 to 97 | 0.22 ± 0.02 | 0.22 ± 0.01 | 0.24 ± 0.03 | 0.25 ± 0.02 | ||
| 114 to 121 | 0.45 ± 0.02 | 0.44 ± 0.04 | 0.73 ± 0.11†† | 0.65 ± 0.06†† | ||
| FFA (mmol.l−1) | ||||||
| Maternal | −61 | 0.54 ± 0.08 | 0.58 ± 0.12 | 0.4 ± 0.13 | 0.46 ± 0.09 | |
| −60 to 30 | 0.35 ± 0.05 | 0.56 ± 0.06** | 0.25 ± 0.02 | 0.62 ± 0.07** | ||
| 40 to 97 | 0.56 ± 0.06 | 0.37 ± 0.06 | 0.43 ± 0.06 | 0.44 ± 0.07 | ||
| 114 to 121 | 0.20 ± 0.03 | 0.19 ± 0.03 | 0.46 ± 0.08†† | 0.37 ± 0.03†† | ||
| Urea (mmoll−1) | ||||||
| Maternal | −61 | 8.39 ± 0.50 | 9.34 ± 0.48 | 8.92 ± 0.61 | 7.21 ± 0.64 | |
| −60 to 30 | 7.46 ± 0.29 | 5.55 ± 0.17** | 7.17 ± 0.27 | 5.40 ± 0.28** | ||
| 40 to 97 | 5.85 ± 0.15 | 5.57 ± 0.19 | 5.57 ± 0.23 | 5.72 ± 0.20 | ||
| 114 to 121 | 5.08 ± 0.33 | 5.13 ± 0.38 | 4.40 ± 0.35 | 4.60 ± 0.21 | ||
| Fetal | 114 to 121 | 5.71 ± 0.29 | 5.76 ± 0.35 | 5.14 ± 0.21†† | 5.42 ± 0.16**†† | |
Day −61 was prior to the start of experiment. Days −60 to 30 were the period of undernutrition. Day 40 to 97 was after undernutrition and prior to surgery. Day 114 to 121 was after surgery. Data are mean ±s.e.m. PCUN, periconceptional undernutrition. See text for details of nutritional groups.
P < 0.01 for twin effect
P < 0.05
P < 0.01 for nutrition effect.
During the undernutrition period, PCUN ewes had lower plasma glucose, insulin and urea, and higher plasma FFA and ketone concentrations than N ewes. Decreased plasma insulin concentrations persisted, but plasma metabolite concentrations were not different from N ewes through the rest of gestation (Table 3). Food intake from day 114 was 7% greater in PCUN than N ewes (2024 ± 74 versus 1884 ± 78 g day−1, P < 0.01).
Fetal
Twin fetuses had lower plasma glucose, insulin and urea concentrations than singletons in late gestation, but there were no significant differences in plasma lactate (data not shown) or IGF-1 concentrations (Table 3).
There were no significant differences between heavy and light twins in plasma glucose, insulin, IGF-1, urea or lactate concentrations (data not shown). The maternal to fetal glucose gradient was less in twins than in singletons (2.46 ± 0.03 versus 2.63 ± 0.03 mmol l−1, P < 0.01).
PCUN resulted in lower fetal plasma glucose and insulin concentrations in singletons, and higher fetal plasma urea concentrations in twins compared with fetuses of N ewes. There were no differences in other plasma metabolite and hormone concentrations between nutritional groups (Table 3). There was no effect of PCUN on the maternal to fetal glucose gradient (2.52 ± 0.03 versus 2.52 ± 0.03 mmol l−1, P = 0.66).
Fetal glucose–insulin axis
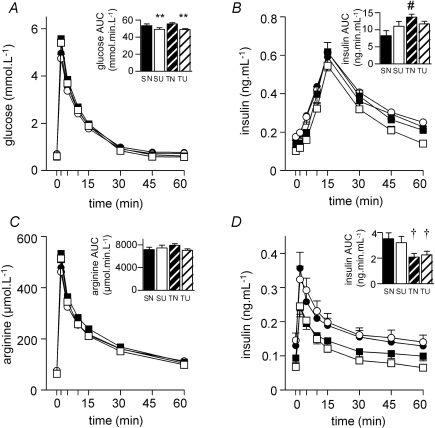
There were no significant differences between singletons and twins in the glucose (Fig. 3A) and arginine (Fig. 3C) AUC in response to the glucose and arginine challenges. However, in fetuses of N ewes, twins had a greater insulin response than singletons to glucose (Fig. 3B) but a lesser insulin response to arginine (Fig. 3D). There were no significant differences in responses to the challenges between heavy and light twins or between members of a twin pair.
Figure 3. Fetal glucose (A) and insulin (B) responses to an intravenous glucose tolerance test; arginine (C) and insulin (D) responses to an intravenous arginine challenge.
Areas under the curve shown as inset histograms. PCUN, periconceptional undernutrition. See text for details of nutritional groups. •, singleton N (SN) (n = 15); ○, singleton PCUN (SU) (n = 13); ▪, twin N (TN) (n = 26); □, twin PCUN (TU) (n = 30). Data are mean ±s.e.m.†P < 0.05 for twin effect; **P < 0.01 for nutrition effect; #P < 0.05 for twin–nutrition interaction.
PCUN resulted in a decreased glucose AUC (Fig. 3A), and abolished the effect of being a twin on insulin response to glucose (Fig. 3B). There was no nutritional effect on arginine AUC (Fig. 3C) or insulin response to arginine (Fig. 3D).
Maternal fast and refeed
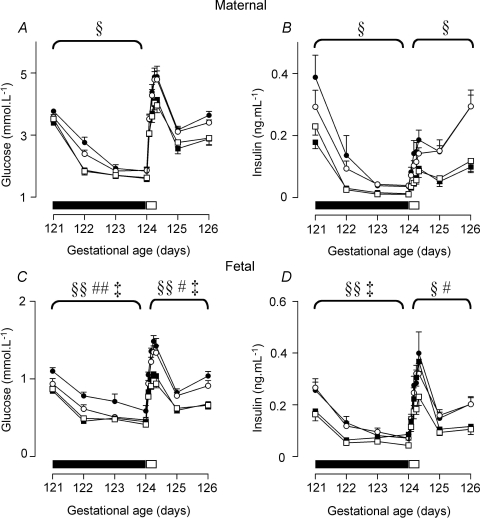
Maternal plasma glucose (Fig. 4A) and insulin concentrations (Fig. 4B) decreased further and faster in response to fasting in twin than in singleton-bearing ewes, and returned to their previously lower concentrations on refeeding. There was no difference between twin and singleton-bearing ewes in the decline of plasma IGF-1 concentrations with fasting (twin-bearing: 82.5 ± 7.0 to 48.3 ± 5.1 ng ml−1; singleton-bearing: 83.3 ± 7.8 to 45.9 ± 5.4 ng ml−1, P < 0.01 for time effect).
Figure 4. Maternal glucose (A) and insulin (B), and fetal glucose (C) and insulin (D) levels during fasting and refeeding.
Fasting period (days 121–124) indicated by filled bar, glucose infusion indicated by open bar. Data are mean ±s.e.m. PCUN, periconceptional undernutrition. See text for details of nutritional groups. •, singleton N (n = 10); ○, singleton PCUN (n = 12); ▪, twin N (n = 12); □, twin PCUN (n = 10). §P < 0.05; §§P < 0.01 for twin × time interaction; #P < 0.05; ##P < 0.01 for twin × nutrition interaction; ‡P < 0.05; ‡‡P < 0.01 for twin × nutrition × time interaction.
Fetal plasma glucose (Fig. 4C) and insulin concentrations (Fig. 4D) decreased further in twin fetuses than in singletons in response to maternal fasting and returned to lower concentrations with refeeding. There was no difference between twin and singletons fetuses in the decline of IGF-1 concentrations with fasting (twins: 78.7 ± 4.2 to 50.5 ± 6.7 ng ml−1, singletons: 69.6 ± 5.7 to 43.1 ± 5.7 ng ml−1, P < 0.01 for time effect).
Maternal plasma glucose concentrations tended to be lower in the fasting period in PCUN than N singleton-bearing ewes (P = 0.07; Fig. 4A), but there was a lesser fall in maternal IGF-1 concentrations in PCUN ewes (PCUN: 60.4 ± 6.1 to 45.5 ± 6.0 ng ml−1, N: 84.6 ± 7.0 to 40.2 ± 4.1 ng ml−1, P = 0.03 for group by time interaction). There was no nutritional effect on maternal insulin concentrations (Fig. 4B).
Fetal plasma glucose and insulin concentrations dropped more in response to maternal fasting in PCUN than N singleton fetuses, and were lower through refeeding (Fig. 4C and D). There was no difference in IGF-1 concentrations between nutritional groups.
Discussion
We hypothesized that if the physiology of twins is largely determined in the periconceptional period, then twinning and PCUN might have similar effects on maternal metabolism, fetal growth and fetal glucose–insulin axis function in late gestation. We found that twin pregnancy led to profound changes in late-gestation fetal growth, fetal glucose–insulin axis function, and maternal metabolism. However, PCUN had different effects on the fetal glucose–insulin axis and on placental morphology from twin pregnancy, and had fewer effects on maternal metabolism and fetal growth. Thus, the physiology of twin pregnancy is quite different from that of singleton pregnancy, and is likely to be determined by a combination of factors acting in both early and late gestation.
Maternal metabolism
We have shown that maternal metabolism in late gestation is different in twin and singleton pregnancy. The decreased food intake of twin-bearing ewes in late gestation may be due to physical restriction of the rumen by the larger mass of twin fetuses. Twin-bearing ewes probably also had reduced tissue stores since, consistent with the findings of another recent study of undernutrition in early gestation (Cleal et al. 2007), their total weights were not different from those of singleton-bearing ewes despite carrying greater conceptus weights. The combination of reduced food intake, reduced tissue stores and the increased metabolic demand imposed by two fetuses (Shinagawa et al. 2005) could together account for the ewes' lower plasma glucose and insulin concentrations, higher plasma FFA and ketone concentrations and the more rapid fall in plasma glucose concentrations with fasting. The smaller increase in maternal plasma glucose concentrations in response to glucose infusion at the end of fasting may also reflect the greater fetal metabolic demand in twin-bearing ewes.
Human studies have also suggested that twin pregnancies are more vulnerable to ‘accelerated starvation’, with rapid development of ketonaemia during fasting (Casele et al. 1996) despite no decrease in food intake (Morley et al. 2006). This is due in part to the greater metabolic demand of twin fetuses, as evidenced by the correlation between maternal glucose disposal rate and conceptus mass (Marconi et al. 1993). Mobilization of fat stores is also an important contributor to maternal nutrition in late pregnancy in both humans and sheep, thus preserving glucose and amino acids for the fetus (Vernon et al. 1981; Butte, 2000). It is unclear whether decreased maternal tissue stores contribute to the ‘accelerated starvation’ in human twin pregnancies.
During periconceptional undernutrition ewes had decreased plasma glucose, insulin and urea concentrations, and increased FFA and ketone concentrations as expected. Except for plasma insulin concentrations, these recovered with refeeding and were not different between PCUN and N ewes in late gestation, despite persistently reduced weight and increased food intake in PCUN ewes. The increased food intake may reflect an attempt to recover tissue stores lost during the period of undernutrition, and at the same time meet the additional metabolic demands of the fetus. When this compensatory strategy was prevented during maternal fasting, we observed a more rapid decrease in glucose concentrations in PCUN ewes, similar to that observed in twin-bearing ewes. However, glucose infusion at the end of the fasting period resulted in maternal plasma glucose profiles that were similar in PCUN and N ewes, suggesting that fetal metabolic demand was not affected by PCUN.
Placenta
Placental mass per fetus was reduced by about a third in twins compared with singletons, although total placental mass was greater. Twin placentae had fewer everted placentomes with predominantly fetal tissue (types C and D), a morphology that may improve oxygen exchange efficiency (Penninga & Longo, 1998), but decrease glucose delivery (Fowden et al. 2006). The maternal to fetal glucose gradient was lower in twins, suggesting either reduced placental glucose consumption or increased efficiency of placental glucose transfer. Reduced utero-placental glucose consumption (Owens et al. 1987a) and increased placental clearance of glucose analogues per unit placental mass (Owens et al. 1987b) have been reported following restriction of placental size by carunclectomy. The placentae of twin fetal sheep at 55 days gestation have increased fetal capillary density and volume (MacLaughlin et al. 2005); thus, the relative reduction in placental size in twin pregnancy may be associated with compensatory changes in placental structure and function in order to ensure adequate substrate supply to the fetus.
Periconceptional undernutrition resulted in a greater proportion of C and D type placentomes, the opposite of the findings in twins, but consistent with other studies of early gestation undernutrition (Steyn et al. 2001; Fowden et al. 2006). Similar changes have also been reported following dexamethasone exposure in early gestation (Laraya et al. 2000) and prolonged hypoxia secondary to altitude (Penninga & Longo, 1998). Everted placentome phenotypes have a greater materno-fetal exchange area (Krebs et al. 1997) suggesting that placental efficiency may be increased, and in rats, even brief changes in maternal nutrition in early pregnancy alter placental function (Jansson et al. 2006; Ericsson et al. 2007). However, placental function has not yet been measured after periconceptional undernutrition in sheep.
Fetal growth
Fetal daily chest girth increment was less in twins than in singletons in late gestation, and twins were smaller and lighter at postmortem. Within a twin pair, the heavy fetus had a greater daily chest girth increment than the light fetus but was already larger at surgery, suggesting that at least some effects of being a twin on fetal growth trajectory are established earlier in gestation. This is consistent with studies in sheep (Harding, 1997b; Bloomfield et al. 2006) and humans (Sebire et al. 1997; Boulot et al. 2000; Bukowski et al. 2007a,b) suggesting that fetal growth trajectory is determined in early pregnancy. The greater impact of maternal fasting on fetal chest girth increment in twins suggests that growth rate in late-gestation twins may be more limited by nutrient supply than it is in singletons, consistent with the altered maternal and fetal metabolism in twins discussed above. A greater slowing of fetal growth trajectory in twin fetuses compared with singleton fetuses during maternal undernutrition has been reported previously (Mellor & Murray, 1982).
In a previous study we showed that singleton fetuses grew more slowly in the 2 weeks prior to term after PCUN (Oliver et al. 2005). The lack of a similar effect of PCUN on late-gestation fetal daily chest girth increment in the current study may be a reflection of the earlier gestation (114–131 versus 126–145 days), as fetal growth slows close to delivery and any differences between groups are likely to be accentuated at this time. Our finding that the lighter twins of PCUN ewes maintained their daily chest girth increment during maternal fasting better than lighter twins of N ewes is, however, consistent with our previous observations that fetal growth in PCUN singletons continued in the face of late-gestation maternal undernutrition, whereas it slowed in N fetuses (Harding, 1997b). We have previously speculated that this reflects some adaptation in feto-placental function induced by PCUN that allows continued fetal growth in the face of limited nutrient supply (Bloomfield et al. 2006). It is not clear why such changes were not seen in singletons in the current study, although the short period of complete fast (3 days) in the current study compared with 10 days of severe undernutrition in the previous study may have limited our ability to detect a difference.
Fetal pancreatic function
The greater insulin response to glucose, but smaller response to arginine, observed in twin fetuses demonstrates altered pancreatic function by 118 days gestation. This pattern may indicate earlier pancreatic maturation in twins resulting in increased β cell responsiveness to glucose, as pancreatic maturation in late gestation is thought to involve apoptosis of amino acid-sensitive fetal β cell and their replacement by glucose-sensitive adult β cells (Scaglia et al. 1997; Fowden et al. 2005). Such a maturational switch could have implications for final β cell number and therefore β cell function in later life. However, this advanced maturation of one organ, the pancreas, would contrast with the reported delayed maturation of the hypothalamic–pituitary–adrenal axis in twin sheep fetuses (McMillen et al. 2004), although organ-specific (Oliver et al. 2002; Bloomfield et al. 2003a), and even tissue-specific (Lillycrop, 2007) responses to intrauterine stimuli are increasingly recognized.
Alternatively, a greater insulin response to glucose challenge could reflect either fetal insulin resistance or reduced suppression of hepatic gluconeogenesis. Insulin resistance would be consistent with reports of an association between impaired glucose tolerance and reduced size at birth in twins (Poulsen et al. 1997; Iliadou et al. 2004), and of insulin resistance in pre-pubertal children (Jefferies et al. 2004). However, it is less consistent with the emerging evidence that insulin sensitivity may be increased in early life and decreases with age in both sheep (Clarke et al. 2000) and humans (Mericq et al. 2005; Poulsen & Vaag, 2006), and that young adult twin sheep have increased insulin sensitivity (Poore et al. 2007).
Reduced suppression of hepatic gluconeogenesis in response to insulin has contributed to impaired insulin action in elderly adult twins (Poulsen & Vaag, 2006). Hepatic gluconeogenesis is thought to be negligible in the fetal sheep and human (Kalhan & Parimi, 2000), although induction of hepatic gluconeogenic enzymes, including the rate-limiting phosphoenolpyruvate carboxykinase (PEPCK) has been reported in chronically hypoglycaemic and hypoinsulinaemic fetal sheep (Narkewicz et al. 1993). In that study, glucose concentrations were reduced by about 50%, compared with the 20% reduction seen in twins in our study. Furthermore, induction of PEPCK is thought to be mediated by cortisol (Fowden et al. 1993), yet adrenal development is delayed in twin fetal sheep (McMillen et al. 2004; Rumball et al. 2007). Thus, either changes in insulin secretory response secondary to pancreatic maturation, or changes in insulin sensitivity would seem to be the most likely mechanisms in our study.
In contrast to the effects of being a twin, PCUN decreased the glucose AUC in response to a glucose challenge, but did not affect responses to arginine challenge. A reduced glucose AUC suggests faster glucose disposal, but this is unlikely to be due to greater insulin sensitivity, since the insulin response was not reduced. Indeed, we have previously shown in singletons that PCUN results in increased fetal insulin secretion in response to a glucose challenge (Oliver et al. 2001), and a similar pattern is present in singletons in this study, although it does not reach statistical significance. Thus, our data suggest that PCUN results in increased glucose disposal by insulin-independent mechanisms, perhaps in part by tissues where glucose uptake is not insulin dependent such as the placenta and fetal brain. Furthermore, the finding that PCUN abolishes the greater insulin response to glucose challenge seen in twins suggests a potential interaction between events in early and late gestation on fetal pancreatic maturation that requires further exploration.
Conclusions
This study demonstrates that the effects of twin pregnancy on the late-gestation sheep fetus have some similarities to the effects of PCUN, but also several differences, and that there is an interaction between the two. It seems likely that the physiology of twin pregnancy is the result of factors operating in both early and late gestation. Furthermore, the maternal metabolic and endocrine environment, fetal and placental growth, and fetal glucose–insulin axis function are all different in twin pregnancies from those in singleton pregnancies, making it essential that twins and singletons are addressed separately in any study of fetal physiology or its postnatal consequences. Although the differences between ovine and human pregnancy mean that these results cannot simply be extrapolated to the human, they do suggest that twins represent another paradigm of the Developmental Origins of Health and Disease. Further exploration of the differences between twin and singleton pregnancy may help to explain the observed inconsistent relationships between birth weight and later disease risk in twins and may provide further insight into some of the mechanisms underlying the Developmental Origins hypothesis.
Acknowledgments
We would like to thank the Health Research Council of New Zealand, the National Research Centre for Growth and Development and the New Zealand Lottery Grants Board for funding this work, and all members of the Fetal Growth Group at the Liggins Institute for their invaluable contributions.
References
- Baird J, Osmond C, MacGregor A, Snieder H, Hales CN, Phillips DIW. Testing the fetal origins hypothesis in twins: the Birmingham twin study. Diabetologia. 2001;44:33–39. doi: 10.1007/s001250051577. [DOI] [PubMed] [Google Scholar]
- Bergvall N, Iliadou A, Johansson S, de Faire U, Kramer MS, Pawitan Y, Pedersen NL, Lichtenstein P, Cnattingius S. Genetic and shared environmental factors do not confound the association between birth weight and hypertension: a study among Swedish twins. Circulation. 2007;115:2931–2938. doi: 10.1161/CIRCULATIONAHA.106.674812. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Giannoulias CD, Gluckman PD, Harding JE, Challis JR. Brief undernutrition in late-gestation sheep programs the hypothalamic-pituitary-adrenal axis in adult offspring. Endocrinology. 2003a;144:2933–2940. doi: 10.1210/en.2003-0189. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Harding JE. The late effects of fetal growth patterns. Arch Dis Child Fetal Neonatal Ed. 2006;91:F299–F304. doi: 10.1136/adc.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Harding JE. Effects of twinning, birth size and postnatal growth on glucose tolerance and hypothalamo-pituitary-adrenal function in post-pubertal sheep. Am J Physiol Endocrinol Metab. 2007;292:E231–E237. doi: 10.1152/ajpendo.00210.2006. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, Challis JRG, Harding JE. A periconceptional nutritional origin for non-infectious preterm birth. Science. 2003b;300:606. doi: 10.1126/science.1080803. [DOI] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, Harding JE, Challis JRG. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology. 2004;145:4278–4285. doi: 10.1210/en.2004-0424. [DOI] [PubMed] [Google Scholar]
- Blum WF, Breier BH. Radioimmunoassays for IGFs and IGFBPs. Growth Regul. 1994;4:11–19. [PubMed] [Google Scholar]
- Boulot P, Vignal J, Vergnes C, Dechaud H, Faure JM, Hedon B. Multifetal reduction of triplets to twins: a prospective comparison of pregnancy outcome. Hum Reprod. 2000;15:1619–1623. doi: 10.1093/humrep/15.7.1619. [DOI] [PubMed] [Google Scholar]
- Bukowski R, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, et al. Fetal growth in early pregnancy and risk of delivering low birth weight infant: prospective cohort study [also see comment] BMJ. 2007a;334:836. doi: 10.1136/bmj.39129.637917.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski R, Smith GC, Malone FD, Ball RH, Nyberg DA, Comstock CH, et al. Human sexual size dimorphism in early pregnancy. Am J Epidemiol. 2007b;165:1216–1218. doi: 10.1093/aje/kwm024. [DOI] [PubMed] [Google Scholar]
- Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256S–1261S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–1099. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- Casele HL, Dooley SL, Metzger BE. Metabolic response to meal eating and extended overnight fast in twin gestation. Am J Obstet Gynecol. 1996;175:917–921. doi: 10.1016/s0002-9378(96)80025-1. [DOI] [PubMed] [Google Scholar]
- Clarke L, Firth K, Heasman L, Juniper DT, Budge H, Stephenson T, Symonds ME. Influence of relative size at birth on growth and glucose homeostasis in twin lambs during juvenile life. Reprod Fertil Dev. 2000;12:69–73. doi: 10.1071/rd99090. [DOI] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Newman JP, Noakes DE, Hanson MA, Green LR. The effect of maternal undernutrition in early gestation on gestation length and fetal and postnatal growth in sheep. Pediatr Res. 2007;62:422–427. doi: 10.1203/PDR.0b013e31813cbe60. [DOI] [PubMed] [Google Scholar]
- Dwyer T, Blizzard L, Morley R, Ponsonby AL. Within pair association between birth weight and blood pressure at age 8 in twins from a cohort study. BMJ. 1999;319:1325–1329. doi: 10.1136/bmj.319.7221.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Saljo K, Sjostrand E, Jansson N, Prasad PD, Powell TL, Jansson T. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007;581:1323–1332. doi: 10.1113/jphysiol.2007.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Gardner DS, Ousey JC, Giussani DA, Forhead AJ. Maturation of pancreatic b-cell function in the fetal horse during late gestation. J Endocrinol. 2005;186:467–473. doi: 10.1677/joe.1.06176. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Mijovic J, Silver M. The effects of cortisol on hepatic and renal gluconeogenic enzyme activities in the sheep fetus during late gestation. J Endocrinol. 1993;137:213–222. doi: 10.1677/joe.0.1370213. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FPB, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572:5–15. doi: 10.1113/jphysiol.2005.104141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JE. Prior growth rate determines the fetal growth response to acute maternal undernutrition in fetal sheep of late gestation. Prenat Neonat Med. 1997a;2:300–309. [Google Scholar]
- Harding JE. Periconceptual nutrition determines the fetal growth response to acute maternal undernutrition in fetal sheep of late gestation. Prenat Neonat Med. 1997b;2:310–319. [Google Scholar]
- Iffy L, Lavenhar MA, Jakobovits A, Kaminetzky HA. The rate of early intrauterine growth in twin gestation. Am J Obstet Gynecol. 1983;146:970–972. doi: 10.1016/0002-9378(83)90976-6. [DOI] [PubMed] [Google Scholar]
- Iliadou A, Cnattingius S, Lichtenstein P. Low birthweight and Type 2 diabetes: a study on 11 162 Swedish twins. Int J Epidemiol. 2004;33:948–953. doi: 10.1093/ije/dyh117. discussion 953–944. [DOI] [PubMed] [Google Scholar]
- Jansson N, Pettersson J, Haafiz A, Ericsson A, Palmberg I, Tranberg M, Ganapathy V, Powell TL, Jansson T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies CA, Hofman PL, Knoblauch H, Luft FC, Robinson EM, Cutfield WS. Insulin resistance in healthy prepubertal twins. J Pediatr. 2004;144:608–613. doi: 10.1016/j.jpeds.2004.01.059. [DOI] [PubMed] [Google Scholar]
- Kalhan S, Parimi P. Gluconeogenesis in the fetus and neonate. Semin Perinatol. 2000;24:94–106. doi: 10.1053/sp.2000.6360. [DOI] [PubMed] [Google Scholar]
- Kenyon PR, Morel PCH, Morris ST. The effect of individual liveweight and condition scores of ewes at mating on reproductive and scanning performance. N Z Vet J. 2004;52:230–235. doi: 10.1080/00480169.2004.36433. [DOI] [PubMed] [Google Scholar]
- Krebs C, Longo LD, Leiser R. Term ovine placental vasculature: comparison of sea level and high altitude conditions by corrosion cast and histomorphometry. Placenta. 1997;18:43–51. doi: 10.1016/s0143-4004(97)90070-9. [DOI] [PubMed] [Google Scholar]
- Laraya NM, Moss TJ, Newnham JP, Challis JRG. Sex-specific changes in proportion of ovine placental subtypes (A,B,C/D) at 0.7 gestation following maternal dexamethasone administration at 0.3 gestation (Abstract A361) J Soc Gynecol Invest. 2000;7:145A. [Google Scholar]
- Levine RS, Hennekens CH, Jesse MJ. Blood pressure in prospective population based cohort of newborn and infant twins. BMJ. 1994;308:298–302. doi: 10.1136/bmj.308.6924.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA. Epigenetics and the influence of maternal diet. Early Hum Dev. 2007;83:S31. [Google Scholar]
- Lithell HO, McKeigue PM, Berglund L, Mohsen R, Lithell UB, Leon DA. Relation of size at birth to non-insulin dependent diabetes and insulin concentrations in men aged 50–60 years. BMJ. 1996;312:406–410. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin SM, Walker SK, Roberts CT, Kleemann DO, McMillen IC. Periconceptional nutrition and the relationship between maternal body weight changes in the periconceptional period and feto-placental growth in the sheep. J Physiol. 2005;565:111–124. doi: 10.1113/jphysiol.2005.084996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Schwartz J, Coulter CL, Edwards LJ. Early embryonic environment, the fetal pituitary-adrenal axis and the timing of parturition. Endocr Res. 2004;30:845–850. doi: 10.1081/erc-200044106. [DOI] [PubMed] [Google Scholar]
- Marconi AM, Davoli E, Cetin I, Lanfranchi A, Zerbe G, Fanelli R, Fennessey PV, Pardi G, Battaglia FC. Impact of conceptus mass on glucose disposal rate in pregnant women. Am J Physiol Endocrinol Metab. 1993;264:E514–E518. doi: 10.1152/ajpendo.1993.264.4.E514. [DOI] [PubMed] [Google Scholar]
- Mellor DJ, Murray L. Effects on the rate of increase in fetal girth of refeeding ewes after short periods of severe undernutrition during late pregnancy. Res Vet Sci. 1982;32:377–382. [PubMed] [Google Scholar]
- Mericq V, Ong KK, Bazaes R, Pena V, Avila A, Salazar T, Soto N, Iniguez G, Dunger DB. Longitudinal changes in insulin sensitivity and secretion from birth to age three years in small- and appropriate-for-gestational-age children. Diabetologia. 2005;48:2609–2614. doi: 10.1007/s00125-005-0036-z. [DOI] [PubMed] [Google Scholar]
- Morley R, Umstad MP, Bond J, Moore VM, Owens JA, Dwyer T, Carlin JB. Maternal dietary intake in twin pregnancies: does it diminish towards term? Twin Res Hum Genet. 2006;9:656–658. doi: 10.1375/183242706778553390. [DOI] [PubMed] [Google Scholar]
- Narkewicz MR, Carver TD, Hay WW., Jr Induction of cytosolic phosphoenolpyruvate carboxykinase in the ovine fetal liver by chronic fetal hypoglycemia and hypoinsulinemia. Pediatr Res. 1993;33:493–496. doi: 10.1203/00006450-199305000-00014. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Breier BH, Gluckman PD, Harding JE. Birth weight rather than maternal nutrition influences glucose tolerance, blood pressure, and IGF-I levels in sheep. Pediatr Res. 2002;52:516–524. doi: 10.1203/00006450-200210000-00010. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Harding JE, Breier BH, Evans PC, Gluckman PD. Glucose but not a mixed amino acid infusion regulates plasma insulin-like growth factor-I concentrations in fetal sheep. Pediatr Res. 1993;34:62–65. doi: 10.1203/00006450-199307000-00015. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Hawkins P, Breier BH, Van Zijl PL, Sargison SA, Harding JE. Maternal undernutrition during the periconceptual period increases plasma taurine levels and insulin response to glucose but not arginine in the late gestational fetal sheep. Endocrinology. 2001;142:4576–4579. doi: 10.1210/endo.142.10.8529. [DOI] [PubMed] [Google Scholar]
- Oliver MH, Hawkins P, Harding JE. Periconceptional undernutrition alters growth trajectory and metabolic and endocrine responses to fasting in late-gestation fetal sheep. Pediatr Res. 2005;57:591–598. doi: 10.1203/01.PDR.0000155942.18096.9C. [DOI] [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Effect of restriction of placental growth on fetal and utero-placental metabolism. J Dev Physiol. 1987a;9:225–238. [PubMed] [Google Scholar]
- Owens JA, Falconer J, Robinson JS. Restriction of placental size in sheep enhances efficiency of placental transfer of antipyrine, 3-O-methyl-D-glucose but not of urea. J Dev Physiol. 1987b;9:457–464. [PubMed] [Google Scholar]
- Penninga L, Longo LD. Ovine placentome morphology: effect of high altitude, long-term hypoxia. Placenta. 1998;19:187–193. doi: 10.1016/s0143-4004(98)90008-x. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Davies MJ, Robinson JS. Fetal growth and the fetal origins hypothesis in twins – problems and perspectives. Twin Res. 2001;4:327–331. doi: 10.1375/1369052012669. [DOI] [PubMed] [Google Scholar]
- Poore KR, Cleal JK, Newman JP, Boullin JP, Noakes DE, Hanson MA, Green LR. Nutritional challenges during development induce sex-specific changes in glucose homeostasis in the adult sheep. Am J Physiol Endocrinol Metab. 2007;292:E32–E39. doi: 10.1152/ajpendo.00253.2006. [DOI] [PubMed] [Google Scholar]
- Poulsen P, Vaag A. The intrauterine environment as reflected by birth size and twin and zygosity status influences insulin action and intracellular glucose metabolism in an age- or time-dependent manner. Diabetes. 2006;55:1819–1825. doi: 10.2337/db05-1462. [DOI] [PubMed] [Google Scholar]
- Poulsen P, Vaag AA, Kyvik KO, Moller Jensen D, Beck-Nielsen H. Low birth weight is associated with NIDDM in discordant monozygotic and dizygotic twin pairs. Diabetologia. 1997;40:439–446. doi: 10.1007/s001250050698. [DOI] [PubMed] [Google Scholar]
- Rumball CWH, Oliver MH, Thorstensen EB, Jaquiery AL, Husted SM, Harding JE, Bloomfield FH. Effects of twinning and periconceptional undernutrition on late-gestation hypothalamic-pituitary-adrenal axis function in ovine pregnancy. Endocrinology. 2007 doi: 10.1210/en.2007-1306. DOI: 10.1210/en.2007-1306. [DOI] [PubMed] [Google Scholar]
- Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/endo.138.4.5069. [DOI] [PubMed] [Google Scholar]
- Sebire NJ, Sherod C, Abbas A, Snijders RJ, Nicolaides KH. Preterm delivery and growth restriction in multifetal pregnancies reduced to twins. Hum Reprod. 1997;12:173–175. doi: 10.1093/humrep/12.1.173. [DOI] [PubMed] [Google Scholar]
- Shinagawa S, Suzuki S, Chihara H, Otsubo Y, Takeshita T, Araki T. Maternal basal metabolic rate in twin pregnancy. Gynecol Obstet Invest. 2005;60:145–148. doi: 10.1159/000086132. [DOI] [PubMed] [Google Scholar]
- Steyn C, Hawkins P, Saito T, Noakes DE, Kingdom JC, Hanson MA. Undernutrition during the first half of gestation increases the predominance of fetal tissue in late-gestation ovine placentomes. Eur J Obstet Gynecol Reprod Biol. 2001;98:165–170. doi: 10.1016/s0301-2115(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Todd SE, Oliver MH, Jaquiery AL, Bloomfield FH, Harding JE. Periconceptional undernutrition of ewes decreases glucose tolerance in their postnatal offspring. Early Hum Dev. 2007;83:S53. [Google Scholar]
- Vatnick I, Schoknecht PA, Darrigrand R, Bell AW. Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol. 1991;15:351–356. [PubMed] [Google Scholar]
- Vernon RG, Clegg RA, Flint DJ. Metabolism of sheep adipose tissue during pregnancy and lactation. Adaptation and regulation. Biochem J. 1981;200:307–314. doi: 10.1042/bj2000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheaton JE, Carlson KM, Windels HF, Johnston LJ. CIDR: a new progesterone-releasing intravaginal device for induction of estrus and cycle control in sheep and goats. Anim Reprod Sci. 1993;33:127–141. [Google Scholar]
- Wust S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30:591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Xu B, Deter RL, Milner LL, Hill RM. Evaluation of twin growth status at birth using individualized growth assessment: comparison with conventional methods. J Clin Ultrasound. 1995;23:277–286. doi: 10.1002/jcu.1870230502. [DOI] [PubMed] [Google Scholar]
- Zhang J, Brenner RA, Klebanoff MA. Differences in birth weight and blood pressure at age 7 years among twins. Am J Epidemiol. 2001;153:779–782. doi: 10.1093/aje/153.8.779. [DOI] [PubMed] [Google Scholar]