Abstract
Injury to the energy-storing superficial digital flexor tendon is common in equine athletes and is age-related. Tenocytes in the superficial digital flexor tendon of adult horses appear to have limited ability to respond adaptively to exercise or prevent the accumulation of strain-induced microdamage. It has been suggested that conditioning exercise should be introduced during the growth period, when tenocytes may be more responsive to increased quantities or intensities of mechanical strain. Tenocytes are linked into networks by gap junctions that allow coordination of synthetic activity and facilitate strain-induced collagen synthesis. We hypothesised that there are reductions in cellular expression of the gap junction proteins connexin (Cx) 43 and 32 during maturation and ageing of the superficial digital flexor tendon that do not occur in the non-injury-prone common digital extensor tendon. Cryosections from the superficial digital flexor tendon and common digital extensor tendon of 5 fetuses, 5 foals (1–6 months), 5 young adults (2–7 years) and 5 old horses (18–33 years) were immunofluorescently labelled and quantitative confocal laser microscopy was performed. Expression of Cx43 and Cx32 protein per tenocyte was significantly higher in the fetal group compared with all other age groups in both tendons. The density of tenocytes was found to be highest in immature tissue. Higher levels of cellularity and connexin protein expression in immature tendons are likely to relate to requirements for tissue remodelling and growth. However, if further studies demonstrate that this correlates with greater gap junctional communication efficiency and synthetic responsiveness to mechanical strain in immature compared with adult tendons, it could support the concept of early introduction of controlled exercise as a means of increasing resistance to later injury.
Keywords: age, connexins (Cx), horses, gap junctions, immunofluorescence, tendons
Introduction
Tenocytes are the cells responsible for synthesis, turnover and repair of the extracellular matrix (ECM) of tendons. Tenocytes are arranged in parallel rows in the longitudinal axis of the tendon, with gap junctions (GJ) linking flattened cytoplasmic processes that extend through the collagenous matrix between cells in the same and adjacent rows. Gap junctions allow the passage of metabolites, ions and small molecules (< 1 kDa) from the cytoplasm of one cell to another (Goldberg et al. 1999; Goodenough & Paul, 2003). These communicating channels connect the tenocytes into a three-dimensional functional network, enabling co-ordinated and appropriate responses to mechanical stimuli including increased collagen synthesis (Merilees & Flint, 1980; Strocchi et al. 1991; McNeilly et al. 1996; Banes et al. 1999).
Gap junctions cluster together in the cytoplasmic membrane to form ‘plaques’, comprising anything from a dozen to several thousand individual channels and ranging from 100 nanometres to several microns in diameter (Forge et al. 1999; Beyer & Berthoud, 2002; Segretain & Falk, 2004). They are dynamic structures with addition of newly synthesised channels to the periphery of plaques as older channels are removed from the centre creating a ‘steady state’ over periods of up to two hours (Gaietta et al. 2002; Lauf et al. 2002). Each individual GJ is composed of two annular hemichannels (connexons) that embed in the cytoplasmic membranes of apposing cells, docking to form a tightly sealed channel (Unger et al. 1999; Falk, 2000). Connexons are comprised of 6 transmembrane proteins termed connexins (Cx); 21 Cx genes have been identified in the human genome, named by the molecular mass (in kDa) of their protein products (Sohl & Willecke, 2004). GJ plaques comprised of Cx32 and Cx43 respectively have been identified in rat, human and avian tendons (McNeilly et al. 1996; Ralphs et al. 1998). These two connexin isoforms do not form heteromeric connexons (Elfgang et al. 1995).
Tendons that store elastic energy to increase the efficiency of high-speed locomotion, including the equine superficial digital flexor tendon (SDFT) and human Achilles tendon (AT), have high rates of injury in athletes that increase with age (Kannus & Jozsa, 1991; Williams et al. 2001). Anatomically opposing tendons that function solely to transmit muscular force and position the limb including the equine common digital extensor tendon (CDET) rarely exhibit strain-induced damage. In many cases tendon rupture is preceded by accumulation of subclinical microdamage that is not repaired by tenocytes including increased amounts of type III collagen, histologically evident matrix degeneration, changes in cellularity and vascularity, and reductions in collagen fibril diameter and crimp angle (Webbon, 1977; Smith et al. 1999; Maffulli et al. 2000; Järvinen et al. 2004). It is known that tenocyte density reduces with age in many tendons, however there has been no comparison of energy-storing and positional tendons during maturation and ageing periods (Webbon, 1977; Goodship et al. 1994; Nakagawa et al. 1994; Crevier-Denoix et al. 1998). A number of studies have indicated dramatic alterations in matrix composition and tenocyte synthetic activity during maturation and with ageing of digital flexor tendons; in some of these studies similar changes were not noted in the CDET (Birch et al. 1999; Perez-Castro & Vogel, 1999; Batson et al. 2003; Goodman et al. 2004). On this basis it has been suggested that horses should begin training during the growth period when the tenocytes may be more synthetically responsive to mechanical strain; this could result in adaptive changes in the matrix that increase resistance to tendon injury in the adult athlete (Smith et al. 1999; Firth, 2006). The synthetic capacity of tenocytes and therefore their ability to repair matrix microdamage is likely to be affected by their potential to communicate via gap junctions; however this has not been defined in any tendon structure. This study addressed the hypothesis that there are significant reductions in tenocyte density and in Cx43 and Cx32 protein expression by the remaining cells during maturation and ageing in the energy-storing equine SDFT that do not occur in the positional and non-injury-prone CDET.
Materials and methods
Specimens
SDFT and CDET specimens were taken with written owner consent from the left forelimbs of 20 horses humanely killed for reasons other than tendon injury. The horses were from the following pre-defined age groups; fetal (full term, non-weight bearing; n = 5), foal (1–6 months; n = 5), young adult (2–7 years; n = 5) and old (18–33 years; n = 5). Segments (0.5 cm) were excised from the core of the mid-metacarpal region of each tendon and snap-frozen in n-hexane pre-cooled in liquid nitrogen. Cryosections (15–20 µm) were cut in the sagittal plane, mounted on poly-L-lysine coated glass slides (VWR, Dorset, UK) and stored at –80 °C.
Immunofluorescent labelling
The cryosections were rehydrated in PBS plus 0.1% (v/v) Tween-20® (Sigma-Aldrich, Dorset, UK) and blocked with PBS containing 5% (v/v) goat serum (Invitrogen, Paisley, UK). Immunolocalisation was performed using standard indirect immunofluorescence, with either monoclonal mouse anti-Cx32 at 10 µg mL−1 (Chemicon International Ltd., Temecula, CA, USA) or mouse anti-Cx43 at 2 µg mL−1 (Chemicon International Ltd.) followed by Alexa488 conjugated goat anti-mouse IgG (7.5 µg mL−1; Invitrogen). Negative control sections were incubated with purified non-immune mouse immunoglobulins (10 or 2 µg mL−1; P.A.R.I.S., Compiegne, France) or with the primary antibody omitted (non-specific negative control). Positive control sections of mouse and equine liver (for Cx32) and heart (for Cx43) were also immunolabelled (Beyer et al. 1987; White et al. 1995). Counterstaining of nuclei was performed using propidium iodide (0.5 µg mL−1; Invitrogen) followed by mounting in Vectashield (Vector Laboratories, Burlingame, CA, USA).
Validation of commercial antibodies for labelling of equine tenocytes
Western blotting was performed for verification of antibody cross-reactivity with equine Cx43 and Cx32 as follows; a midmetacarpal SDFT segment (2 cm) was excised from the forelimb of a 5-month-old foal and cut into 2 mm3 cubes. The cubes were added to Dulbecco's Modified Eagle Media (4500 mg L−1 D-glucose, 25 mM HEPES with L-glutamine; Invitrogen) supplemented with 10% fetal bovine serum (FBS) and antibiotics (1% of 5000 U mL−1 penicillin and 5000 U mL−1 streptomycin; Invitrogen) prepared with 1 mg mL−1 bacterial collagenase type VIII (Sigma-Aldrich) solution. The solution was incubated under standard culture conditions (37 °C in a humidified atmosphere of 5% carbon dioxide and 95% air) for 12 h (overnight) to digest the tendon matrix. Samples were then strained through a 40 µm cell strainer and cells harvested by centrifugation. Cell pellets were resuspended in fresh media and grown to confluence. Cultured tenocytes were placed into reducing sample buffer and sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was performed on a 10 % gel. For western blotting, proteins were transferred to Hybond™ Electrochemiluminescence (ECL) nitrocellulose membranes (Amersham Biosciences, Bucks, UK). Membranes were incubated with primary antibodies (Cx32 at 1.0 µg mL−1; Cx43 at 0.5 µg mL−1) diluted in PBS. Indirect immunoperoxidase staining was performed using goat anti-mouse IgG horseradish peroxidase (HRP)-labelled antibody solution (1:1000; Dako, Glostrup, Denmark). Antigen-antibody complexes were visualized by chemiluminescence detection (ECL Western blotting detection reagents, Amersham Biosciences).
Quantitative confocal laser scanning microscopy (CLSM)
Sections were examined with a Leica SP2 AOBS (Leica Microsystems, Milton Keynes, UK) set up for dual channel fluorescence using fluorescein (FITC) and rhodamine (TRITC) filter settings (laser wavelengths 488 nm and 543 nm respectively). Images were taken using a 20x lens (NA 0.70) and separated with prismatic separation (user optimised) for each fluorophore. Two cryosections were stained for each of Cx32 and Cx43 from each horse. Two representative fields of view were chosen in each of the cryosections, taking care to avoid interfascicular (endotenon) regions, and thirty successive serial images (z-stack) were taken through a depth of 10 µm for each field. For each field of view (total of 4 per tendon specimen), the fifth and twenty-fifth serial sections were analysed respectively, totalling eight images for each horse for Cx32 and eight for Cx43. A power calculation with preliminary Cx plaque number and area data from a fetal and an 18-year-old horse was performed to determine that a minimum of 5 fields were required for statistical significance. Images were imported into imaging software where they were converted from a palette image to a 24-bit RGB file to enable recognition of format (Image Pro Plus 5.0, Silver Spring, Media Cybernetics, MD, USA). Automated counting was achieved by selecting an appropriate pixel intensity range for tenocytes and for Cx respectively, by segmentation. Segmentation is a process by which certain colours in an image are visually identified and then isolated from the image as a whole. Segmented areas were masked (pseudo-coloured) with another colour to improve the ability to distinguish stained regions, therefore improving accuracy of counting and measuring. Determination of optimum intensity of pixel colour of connexin labelling was conducted at the beginning of analysis from samples of various ages from the SDFT and CDET, stained with both Cx32 and Cx43.
A defined area of interest box (AOI; 402 × 402 µm, total of 1.29 mm2 for 8 AOI) was added to the centre of each image obtained, and tenocyte nuclei and Cx plaques counted within it. By common convention, nuclei and Cx plaques touching the north or east borders were discounted and those touching south or west borders were included. The AOI was placed in the same location of each image obtained. This entire procedure was written into user-designed macros (one for each: tenocyte and nuclei) to ensure standardisation of protocol. The images were calibrated using scale bars taken from the confocal images. The total area (µm2) and number of Cx plaques was calculated and divided by the number of tenocyte nuclei in 1 mm2 (tenocyte density) for each horse.
Statistical analysis
Kolmogorov-Smirnov tests (SPSS software, Chicago, IL, USA) for normality were performed for all data sets prior to comparative analysis. The mean values for individual horses were used to calculate total areas and numbers of Cx plaques per tenocyte nucleus in each age group. Differences in tenocyte density and in numbers, total areas of Cx43 and Cx32 per tenocyte nucleus and Cx43:Cx32 ratios for both tendons (SDFT and CDET) between age groups (fetal, foal, young adult and old) were tested for statistical significance using a general linear model, followed by post hoc bonferroni adjustment of p values (SAS software, SAS Institute, Cary, NC, USA). Statistical significance level was set at P < 0.05. Bonferroni post hoc tests give greatest control of type I errors and are more powerful compared with other routine tests when comparisons are small.
Results
Maturational and tendon-related differences in tenocyte density
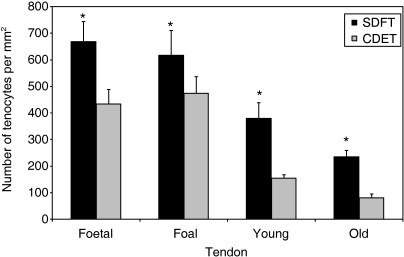
Tenocyte nuclei in the immature horses (fetal and foal age groups) were rounder than those in the adult horses (young adult and old age groups), and arrangement of the nuclei in parallel rows in the longitudinal axis was not as clearly defined. Mean tenocyte density was significantly greater in the immature than the adult horses (P ≤ 0.0001) but there were no differences between fetal and foal groups or between young adult and old groups respectively (Fig. 1). There was a significantly higher tenocyte density in the SDFT than the CDET in all four age groups (P < 0.0001).
Fig. 1.
Tenocyte density (expressed as number per mm2) in the SDFT and CDET from each age group. Asterisk indicates a significant difference compared to the CDET. Error bars are indicated as ±SEM. SDFT, superficial digital flexor tendon; CDET, common digital extensor tendon.
Maturational differences in connexin plaque expression
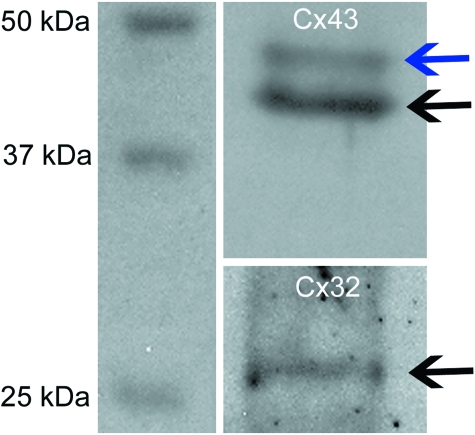
Western blotting using mouse anti-Cx antibodies confirmed cross-reaction with Cx protein from cultured equine tenocytes. Antibodies bound to the appropriately sized proteins for Cx32 and Cx43 isoforms, to give 27 KDa (Cx32) or 43–47 KDa (Cx43) bands (Fig. 2). The two bands for Cx43 are likely a reflection of different states of phosphorylation.
Fig. 2.
Western blots showing bands for Cx43 (43 kDa, black arrow, plus a band usually identified at 47kDa indicating different states of phosphorylation, blue arrow) and (b) Cx32 (27 kDa, black arrow). Cx32, connexin 32; Cx43, connexin 43.
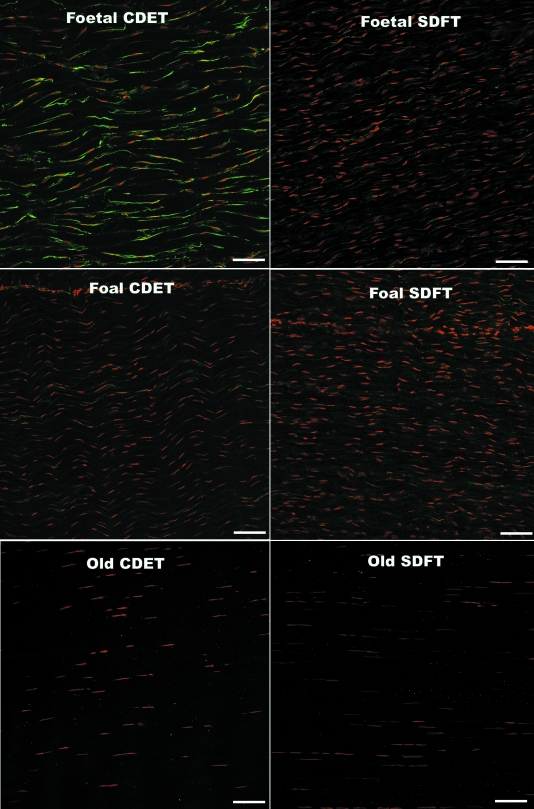
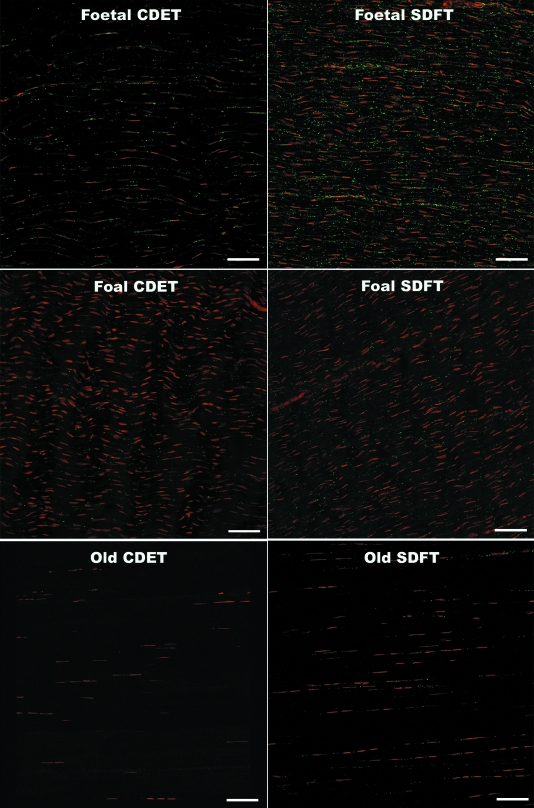
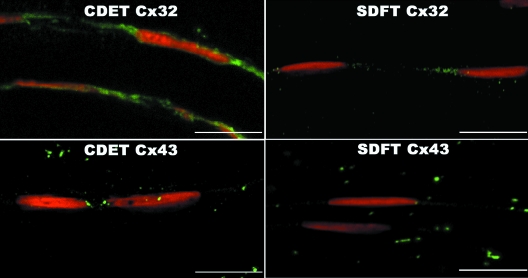
Confocal laser scanning microscopy (CLSM) of immunofluorescently labelled sections revealed rows of tenocyte nuclei within the tendon matrix, with closely located connexin plaques. Negative control sections did not reveal any non-specific staining of the secondary antibody (data not shown). Labelling of Cx32 and Cx43 plaques and tenocyte nuclei was well-defined, enabling accurate measurements using automated computerised image analysis. The expression of the Cx32 and Cx43 plaques differed significantly between fetal and all older age groups. In the fetal SDFT and CDET, Cx32 labelling covered large areas of the tenocyte nuclear surfaces in the form of long thin regions of cytoplasmic staining (mean area 1.1 ± 0.3 µm2) (Fig. 3). The mean areas of labelled Cx32 protein regions appeared to be greater in the fetal CDET than in the SDFT when considering individual horses (Fig. 3), however this difference was not statistically significant when comparing groups (P = 0.57). The extensive regions of positive Cx32 labelling in fetal tendon were interpreted as indicating large amounts of intracytoplasmic protein in transit to the cytoplasmic membrane. Immunolabelled Cx32 protein in mature horses and Cx43 protein in all age groups, formed smaller, more discrete, punctate foci (plaques) closely adjacent to tenocyte nuclei (between tenocytes of the same row) (Figs 3 and 4). Cx43 plaques were also noted within the matrix between nuclear rows (between tenocytes in adjacent rows). Images taken at higher magnification demonstrate the punctuate nature of Cx43 labelling and cytoplasmic labelling of Cx32 in fetal tendon (Fig. 5).
Fig. 3.
Longitudinal cryosections from the SDFT and CDET immunolabelled with Cx32 (green plaques) from a fetus, foal and an old horse. Tenocyte nuclei counterstained with propidium iodide (red). Bar = 80 µm.
Fig. 4.
Longitudinal cryosections from the SDFT and CDET immunolabelled with Cx43 (green plaques) from a fetus, foal and an old horse. Tenocyte nuclei counterstained with propidium iodide (red). Bar = 80 µm.
Fig. 5.
High magnification of longitudinal cryosections from a fetus. SDFT and CDET immunolabelled with Cx32 and Cx43. Tenocyte nuclei counterstained with propidium iodide. Bar = 15 µm.
Maturational changes in Cx32 and Cx43 plaque total area and number per tenocyte nucleus
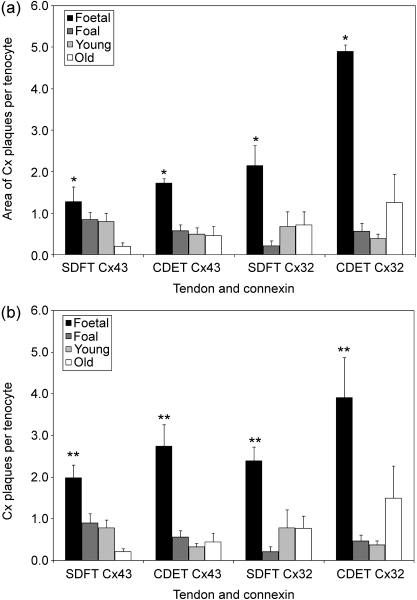
The total immunolabelled Cx protein area per tenocyte was significantly higher in the fetal group than any of the other groups (foal, young adult or old) for both Cx43 (P < 0.01) and Cx32 (P < 0.02) in the SDFT and in the CDET (Fig. 6a). This parameter did not differ significantly between the SDFT and CDET. Similarly, the numbers of Cx plaques per tenocyte were significantly higher in the fetal group than any other group for both Cx43 (P < 0.0001) and Cx32 (P < 0.02), again with no tendon-specific difference (Fig. 6b). An increase in the number and area of Cx32 plaques in the CDET of old horses compared with adult horses was noted, however this difference between them was not statistically significant (P = 0.189). Similarly there were apparent reductions in Cx43 plaque number and area per tenocyte between adult and old horses in the SDFT, however these changes were also not statistically significant (P = 0.58, P = 0.24). There were no significant differences in number or area of Cx43:Cx32 plaque ratios in any of the age groups for either tendon.
Fig. 6.
(a) Total area of Cx32 and Cx43 plaques per tenocyte (expressed per µm2) in the SDFT and CDET from each age group. Asterisk indicates a significant difference compared with all other age groups. The mean area of Cx plaques per tenocyte was significantly higher in the fetal group than in any other group for both Cx43 and Cx32, but did not differ between the SDFT and CDET. Error bars are indicated as ±SEM. (b) Cx32 and Cx43 plaque number per tenocyte (expressed per mm2) in the SDFT and CDET from each age group. Double asterisk indicates a significant difference compared with all other age groups. The number of Cx plaques was significantly higher in the fetal group than in any other group for both Cx43 and Cx32, but did not differ between tendons.
Discussion
Changes in tenocyte connexin expression during maturation
This study supported the hypothesis that significant reductions in tenocyte density and Cx protein expression occur during digital tendon maturation in the horse, however no significant changes were noted with ageing (between young adult and old groups). The relatively high tenocyte densities and amounts of Cx protein per cell in tendons of late-term fetuses were not surprising, given the need for the cellular population to facilitate rapid adaptive alterations in matrix components with the first few days of birth as foals stand and walk (Koterba, 1990; Lin et al. 2005). However, the significant reductions in Cx43 and Cx32 protein expression per tenocyte that followed within the first few months of life (between fetal and foal groups) in both tendons were not predicted. Tenocyte GJ communication should be important for coordination and facilitation of cellular proliferation and/or matrix synthesis as tendons grow (Becker & Mobbs, 1999). The cross-sectional area of the SDFT more than doubles between 50 and 365 days of age and increases in bodyweight are most rapid within the first 90 days of birth (Hintz et al. 1979; Kasashima et al. 2002). Similar data for this period for the cross-sectional area of the CDET is not available. Strain is known to upregulate collagen synthesis and Cx43 protein expression by avian tendon cells, with significant reduction of the collagen synthetic response by treatment with chemical GJ blockers (Banes et al. 1996; Banes et al. 1999). However, tendon strains do not necessarily increase with body size as in adult horses due to changes in anatomical proportions and tendon properties including elastic modulus during growth (Bullimore & Burn, 2006).
It is also possible that other Cx isoforms are present that have more important roles in facilitation of matrix synthesis. Protein expression of Cx26, Cx42, Cx45 and Cx45.6 has been demonstrated in avian tendon (Tsuzaki et al. 1997). In one study some degree of intercellular communication remained when avian tenocytes were treated with antisense oligodeoxynucleotides (asODN) to both Cx43 and Cx32. This was proposed to be due to the presence of GJ comprised of other Cx isoforms, however complete knockdown of Cx43 and Cx32 protein expression respectively was not achieved in that system (Waggett et al. 2006). There is some evidence that Cx isoforms can differentially affect strain-induced collagen synthesis; the study involving asODN treatment of avian tenocytes indicated that Cx32 and Cx43 were positive and negative regulators respectively within that system (Waggett et al. 2006). Relative amounts of Cx43 and Cx32 protein did not differ significantly with maturation or ageing or between tendons in the current study, however there was significant individual variation that could be important. However, as tissues in different species will not necessarily express the same Cx proteins, and expression can alter during development or in cell culture (Arita et al. 2002; Yamaoka et al. 2002), the Cx isoform expression patterns in tendons of immature and adult horses and specific effects of any further Cx proteins detected on strain-induced collagen synthesis, requires further study. Additionally, any potential differential effects of various Cx isoforms on relative expression of different types of collagen by any cell type have not been studied. Tenocytes in degenerate and ageing tendons have been shown to upregulate expression of type III collagen, that is thought to be associated with weakening of the matrix and thus increased susceptibility to injury (Birch et al. 1999; Maffulli et al. 2000).
In the current investigation differences in GJ communication between age groups and tendons were not directly measured, and require further investigation. Numbers and total areas of Cx plaques per tenocyte do not necessarily have simple and clearly defined relationships with GJ communication efficiency. Gap junction plaques are complex and dynamic structures, and various numbers of their component GJ channels may be open or closed at any one time (Brink, 2002). Significant decreases in Cx protein expression in some other cell types including ageing astrocytes and mammary epithelial cells with different levels of activity, have not been associated with reductions in cell coupling (Sia et al. 1999; Cotrina et al. 2001).
Changes in tenocyte density during maturation
The measurement of higher tenocyte densities in tendons of immature horses compared with adults is in agreement with a previous study of the equine SDFT (Crevier-Denoix et al. 1998), however in the current investigation tenocyte numbers were quantified rather than graded. Our measurements were not in agreement with a study in which DNA concentration in the SDFT was shown to decrease following birth and then increase again (Lin et al. 2005). However, measurement of DNA to estimate cellularity will include cells in the endotendon (interfascicular tissue) including fibroblasts and cells comprising vascular walls; such measurements are therefore not directly comparable with analysis of tenocytes identified visually within fascicles in cryosections. The mechanisms by which tenocyte numbers reduce during maturation have not been defined. The significant increases in SDFT cross-sectional area during growth result in tenocyte rows becoming more widely spaced due to deposition of matrix between them. Cell death may also play a role but this has not been quantified in equine tendons at different stages of maturation; low numbers of apoptotic cells have been documented in the normal adult SDFT only (Hosaka et al. 2005). Evidence of apoptosis in the form of fragmented nuclei or nuclear debris was not noted in microscopic fields analysed for this study. However, the significant reduction in cellularity was shown to occur between foals (1–6 months) and young adults (2–7 years) with no analysis of samples from horses between 6 months and 2 years of age. If there is significant cell death occurring in growing tendon the effect of this on GJ-connected cellular networks cannot currently be predicted as this might depend on whether tenocytes are lost individually or, for example, as entire rows. In other developing tissues it has been shown that gap junctions may mediate the death of adjacent cells (the ‘bystander effect’), however in certain situations GJ communication may also increase cell survival (the ‘good Samaritan effect’) (Andrade-Rozental et al. 2000; Cusato et al. 2003). It is not known how apoptosis of certain individual cells or groups of cells in tenocyte networks might affect their overall synthetic capacity. Numbers of cells passing through the cell cycle, particularly in immature tendons, may also need to considered; connexin proteins have been shown to positively regulate cell proliferation in other developing tissues (Becker & Mobbs, 1999). This could be one factor in the downregulation of cellular expression of Cx43 and Cx32 as equine tendons mature. The level of cell proliferation in immature equine tendon is not known, however mitotic figures have not been noted in histological sections of specimens from the age groups in this study (Patterson-Kane and Stanley, personal observation).
One previous study indicated low collagen turnover in the adult SDFT (Birch et al. 1998; Batson et al. 2003), and tenocytes derived from the SDFT of adult horses showed lower levels of strain-induced collagen synthesis than those from foal tendons (Goodman et al. 2004). It has been suggested that the reason for low cellular synthetic activity in the adult SDFT is to maintain the matrix of this energy-storing tendon within narrow optimal limits of elasticity (Smith et al. 2002). In the current study, although the tenocyte density reduced significantly between foal and young adult groups, the expression of Cx43 and Cx32 plaque protein per remaining cell was maintained at the same level. These findings could imply a lower potential for coordinated synthetic and reparative tenocyte activity in digital tendons of adult compared with immature horses. Further studies are required to determine whether tenocytes in immature tendons, particularly in the immediate postnatal period, show more appropriate synthetic responses to mechanical strain and matrix damage than those in adult tendons. If this proves to be the case it would strengthen support for beginning conditioning exercise in younger horses to increase later resistance to injury (Smith et al. 1999; Firth, 2006).
Tendon-specific differences in tenocyte density
Tenocyte densities of the SDFT and CDET from immature and adult horses had not previously been compared. In all four age groups the CDET was significantly less cellular than the SDFT, and the level of reduction in tenocyte density between immature and adult horses was greater. No age-related differences in tenocyte density were measured in either tendon. This did not agree with a previous study of adult horses in which age-related reductions in mean DNA concentration were documented in the SDFT but not the CDET (Batson et al. 2003). However, as stated previously, analysis of DNA extracted from all cell types in a tendon specimen will not necessarily correlate with analysis of the tenocyte population only as in the current study. Differences between the SDFT and CDET in the fetal group support a previous assertion that equine tendons are not biochemically ‘blank’ at birth (Lin et al. 2005). The lower tenocyte density in the CDET than the SDFT in all age groups most likely relates to the differing functions of these tendons. The SDFT in the galloping horse experiences high strains in order to store sufficient amounts of energy, and as a result works within extremely narrow mechanical safety margins (Stephens et al. 1989; Wilson et al. 1991). The anatomically opposing CDET as a positional structure experiences lower peak strains and rarely suffers strain-induced injury (Kear & Smith, 1975; McIlwraith, 2002). As tenocytes are believed to routinely repair matrix microdamage to prevent fatigue-induced tendon failure (Ker, 2002; Noble, 2003) this may explain their (pre-programmed) higher density in the energy-storing SDFT. There was an apparent increase in Cx32 protein expression by tenocytes in the CDET of old horses, but this change did not prove to be statistically significant even when analysed separately (P = 0.189). However, the old group had a large range of ages and most likely high variability in past exercise histories resulting in a relatively large standard deviation. Connexin 32 only occurs in GJ between tenocytes in the same row (McNeilly et al. 1996) and unlike SDFT cells, tenocytes in the CDET may retain the synthetic capacity to upregulate it as the rows become more isolated from each other. Further study using more narrowly defined age groups will be necessary to determine if these changes are representative findings.
In summary, significant reductions in tenocyte density occur between immature and adult equine digital tendons, with reductions in Cx43 and Cx32 protein expression early in the maturation period. The high level of cellular expression of these connexin isoforms in fetal tendons reduces significantly within the first few months of life. Similar maturational changes were measured in the SDFT and the less cellular, non-injury-prone CDET. Although relatively high levels of cellularity and Cx protein expression in immature tendons most likely relate to developmental requirements, the tenocyte networks at that stage might have a greater ability to respond adaptively to exercise. This would support recent, controversial suggestions that there is a ‘window of opportunity’ during equine tendon development during which controlled exercise regimens could result in increased resistance to injury at a later date. Studies of maturational and age-related changes in the efficiency of tenocyte GJ intercellular communication and effects of this on strain-induced collagen synthesis are ongoing.
References
- Andrade-Rozental AF, Rozental R, Hopperstad MG, Wu JK, Vrionis FD, Spray DC. Gap junctions: the ‘kiss of death’ and the ‘kiss of life’. Brain Res Rev. 2000;32:308–315. doi: 10.1016/s0165-0173(99)00099-5. [DOI] [PubMed] [Google Scholar]
- Arita K, Akiyama M, Tsuji Y, et al. Changes in gap junction distribution and connexin expression pattern during human fetal skin development. J Histochem Cytochem. 2002;50:1493–500. doi: 10.1177/002215540205001109. [DOI] [PubMed] [Google Scholar]
- Banes AJ, Tsuzaki M, Yamamoto J, et al. Trans Orthop Res Soc. Vol. 21. Atlanta: 1996. Connexin expression is upregulated by mechanical load in avian and human tendon cells; pp. 3–1. [Google Scholar]
- Banes AJ, Weinhold P, Yang X, et al. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin Orthop. 1999:356–370. doi: 10.1097/00003086-199910001-00034. [DOI] [PubMed] [Google Scholar]
- Batson EL, Paramour RJ, Smith TJ, et al. Are the material properties and matrix composition of equine flexor and extensor tendons determined by their functions? Equine Vet J. 2003;35:314–318. doi: 10.2746/042516403776148327. [DOI] [PubMed] [Google Scholar]
- Becker DL, Mobbs P. Connexin alpha1 and cell proliferation in the developing chick retina. Exp Neurol. 1999;156:326–332. doi: 10.1006/exnr.1999.7027. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Berthoud VM. Gap junction synthesis and degredation as therapeutic targets. Current Drug Targets. 2002;3:409–416. doi: 10.2174/1389450023347245. [DOI] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch HL, Bailey AJ, Goodship AE. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in the extracellular matrix composition. Equine Vet J. 1998;30:534–539. doi: 10.1111/j.2042-3306.1998.tb04530.x. [DOI] [PubMed] [Google Scholar]
- Birch HL, Bailey JV, Bailey AJ, Goodship AE. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet J. 1999;31:391–396. doi: 10.1111/j.2042-3306.1999.tb03838.x. [DOI] [PubMed] [Google Scholar]
- Brink PR. Are gap junction channels a therapeutic target and if so what properties are best exploited? Curr Drug Targets. 2002;3:417–425. doi: 10.2174/1389450023347326. [DOI] [PubMed] [Google Scholar]
- Bullimore SR, Burn JF. Dynamically similar locomotion in horses. J Exp Biol. 2006;209:455–465. doi: 10.1242/jeb.02029. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Gao Q, Lin JH, Nedergaard M. Expression and function of astrocytic gap junctions in aging. Brain Res. 2001;901:55–61. doi: 10.1016/s0006-8993(01)02258-2. [DOI] [PubMed] [Google Scholar]
- Crevier-Denoix N, Collobert C, Sanaa M, et al. Mechanical correlations derived from segmental histologic study of the equine superficial digital flexor tendon, from foal to adult. Am J Vet Res. 1998;59:969–977. [PubMed] [Google Scholar]
- Cusato K, Bosco A, Rozental R, et al. Gap junctions mediate bystander cell death in developing retina. J Neurosci. 2003;23:6413–6422. doi: 10.1523/JNEUROSCI.23-16-06413.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfgang C, Eckert R, Lichtenberg-Frate H, et al. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol. 1995;129:805–817. doi: 10.1083/jcb.129.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk MM. Biosynthesis and structural composition of gap junction intercellular membrane channels. Eur J Cell Biol. 2000;79:564–574. doi: 10.1078/0171-9335-00080. [DOI] [PubMed] [Google Scholar]
- Firth EC. The response of bone, articular cartilage and tendon to exercise in the horse. J Anat. 2006;208:513–526. doi: 10.1111/j.1469-7580.2006.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A, Becker D, Casalotti S, et al. Gap junctions and connexin expression in the inner ear. Novartis Found Symp. 1999;219:134–50. doi: 10.1002/9780470515587.ch9. discussion 151–156. [DOI] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, et al. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503–507. doi: 10.1126/science.1068793. [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Goodman SA, May SA, Heinegard D, Smith RK. Tenocyte response to cyclical strain and transforming growth factor beta is dependent upon age and site of origin. Biorheology. 2004;41:613–628. [PubMed] [Google Scholar]
- Goodship AE, Birch HL, Wilson AM. The pathobiology and repair of tendon and ligament injury. Vet Clin North Am Equine Pract. 1994;10:323–349. doi: 10.1016/s0749-0739(17)30359-0. [DOI] [PubMed] [Google Scholar]
- Hintz HF, Hintz RL, Van Vleck LD. Growth rate of thoroughbreds, effect of age of dam, year and month of birth, and sex of foal. J Anim Sci. 1979;48:480–487. doi: 10.2527/jas1979.483480x. [DOI] [PubMed] [Google Scholar]
- Hosaka Y, Teraoka H, Yamamoto E, Ueda H, Takehana K. Mechanism of cell death in inflamed superficial digital flexor tendon in the horse. J Comp Pathol. 2005;132:51–58. doi: 10.1016/j.jcpa.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Järvinen TAH, Järvinen TLN, Kannus P, Józsa L, Järvinen M. Collagen fibres of spontaneously ruptured human tendons display decreased thickness and crimp angle. J Orthop Res. 2004;22:1303–1309. doi: 10.1016/j.orthres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–1525. [PubMed] [Google Scholar]
- Kasashima Y, Smith RK, Birch HL, et al. Exercise-induced tendon hypertrophy: cross-sectional area changes during growth are influenced by exercise. Equine Vet J Suppl. 2002:264–268. doi: 10.1111/j.2042-3306.2002.tb05430.x. [DOI] [PubMed] [Google Scholar]
- Kear M, Smith RN. A method for recording tendon strain in sheep during locomotion. Acta Orthop Scand. 1975;46:896–905. doi: 10.3109/17453677508989277. [DOI] [PubMed] [Google Scholar]
- Ker RF. The implications of the adaptable fatigue quality of tendons for their construction, repair and function. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:987–1000. doi: 10.1016/s1095-6433(02)00171-x. [DOI] [PubMed] [Google Scholar]
- Koterba AM. Physical examination. In: Korterba AM, Drummond WH, Kosch PC, editors. Equine clinical neonatology. Baltimore: Lippincott Williams & Wilkins; 1990. pp. 71–79. [Google Scholar]
- Lauf U, Giepmans BN, Lopez P, et al. Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA. 2002;99:10446–10451. doi: 10.1073/pnas.162055899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YL, Brama PA, Kiers GH, DeGroot J, van Weeren PR. Functional adaptation through changes in regional biochemical characteristics during maturation of equine superficial digital flexor tendons. Am J Vet Res. 2005;66:1623–1629. doi: 10.2460/ajvr.2005.66.1623. [DOI] [PubMed] [Google Scholar]
- Maffulli N, Ewen SWB, Waterson SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic Achilles tendons produce greater quantities of type III collagen than tenocytes from normal tendons. An in vitromodel of human tendon healing. Am J Sports Med. 2000;28:499–505. doi: 10.1177/03635465000280040901. [DOI] [PubMed] [Google Scholar]
- McIlwraith CW. Diseases of the joints, tendons, ligaments and related structures. In: Stashak TS, editor. Adams’ lameness in horses. 5. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 594–640. [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Merilees MJ, Flint MH. Ultrastructural study of tensional pressure zones in rabbit flexor tendons. J Anat. 1980;153:87–106. doi: 10.1002/aja.1001570109. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Majima T, Nagashima K. Effect of ageing on ultrastructure of slow and fast skeletal muscle tendon in rabbit Achilles tendons. Acta Physiol Scand. 1994;152:307–313. doi: 10.1111/j.1748-1716.1994.tb09810.x. [DOI] [PubMed] [Google Scholar]
- Noble B. Bone microdamage and cell apoptosis. Eur Cell Mater. 2003;6:46–55. doi: 10.22203/ecm.v006a05. [DOI] [PubMed] [Google Scholar]
- Perez-Castro AV, Vogel KG. In situ expression of collagen and proteoglycan genes during development of fibrocartilage in bovine deep flexor tendon. J Orthop Res. 1999;17:139–148. doi: 10.1002/jor.1100170120. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Benjamin M, Waggett AD, et al. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat. 1998;193:215–222. doi: 10.1046/j.1469-7580.1998.19320215.x. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segretain D, Falk MM. Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta. 2004;1662:3–21. doi: 10.1016/j.bbamem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sia MA, Woodward TL, Turner JD, Laird DW. Quiescent mammary epithelial cells have reduced connexin43 but maintain a high level of gap junction intercellular communication. Dev Genet. 1999;24:111–122. doi: 10.1002/(SICI)1520-6408(1999)24:1/2<111::AID-DVG11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Smith RKW, Birch H, Patterson-Kane J, et al. Should equine athletes commence training during skeletal development?: changes in tendon matrix associated with development, ageing, function and exercise. Equine Vet J Suppl. 1999;30:201–209. doi: 10.1111/j.2042-3306.1999.tb05218.x. [DOI] [PubMed] [Google Scholar]
- Smith RKW, Birch HL, Goodman S, Heinegard D, Goodship AE. The influence of ageing and exercise on tendon growth and degeneration-hypotheses for the initiation and prevention of strain-induced tendinopathies. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:1039–1050. doi: 10.1016/s1095-6433(02)00148-4. [DOI] [PubMed] [Google Scholar]
- Sohl G, Willecke K. Gap junctions and the connexin protein family. Cardiovasc Res. 2004;62:228–232. doi: 10.1016/j.cardiores.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Stephens PR, Nunamaker DM, Butterweck DM. Application of a Hall-effect transducer for measurement of tendon strains in horses. Am J Vet Res. 1989;50:1089–1095. [PubMed] [Google Scholar]
- Strocchi R, De Pasquale V, Guizzardi S, et al. Human Achilles Tendon: Morphological and Morphometric Variations as a function of age. Foot and Ankle. 1991;12:100–104. doi: 10.1177/107110079101200207. [DOI] [PubMed] [Google Scholar]
- Tsuzaki M, Yang X, Burt J, et al. Trans Orthop Res Soc. Vol. 22. San Francisco: 1997. Avian tendon cells express multiple connexins but acute mechanical load reduces cell-cell coupling; p. 712. [Google Scholar]
- Unger VM, Kumar NM, Gilula NB, Yeager M. Three-dimensional structure of a recombinant gap junction membrane channel. Science. 1999;283:1176–1180. doi: 10.1126/science.283.5405.1176. [DOI] [PubMed] [Google Scholar]
- Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145–1154. doi: 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Webbon PM. A post mortem study of equine digital flexor tendons. Equine Vet J. 1977;9:61–67. doi: 10.1111/j.2042-3306.1977.tb03981.x. [DOI] [PubMed] [Google Scholar]
- White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol Biol Cell. 1995;6:459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RB, Harkins LS, Hammond CJ, Wood JL. Racehorse injuries, clinical problems and fatalities recorded on British racecourses from flat racing and National Hunt racing during 1996, 1997 and 1998. Equine Vet J. 2001;33:478–486. doi: 10.2746/042516401776254808. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Baker GJ, Pijanowski GJ, Boero MJ, Badertscher RR. Composition and morphologic features of the interosseous muscle in Standardbreds and Thoroughbreds. Am J Vet Res. 1991;52:133–139. [PubMed] [Google Scholar]
- Yamaoka Y, Sawa Y, Ebata N, Ibuki N, Yoshida S. Cultured periodontal ligament fibroblasts express diverse connexins. Tissue Cell. 2002;34:375–380. doi: 10.1016/s0040816602000381. [DOI] [PubMed] [Google Scholar]