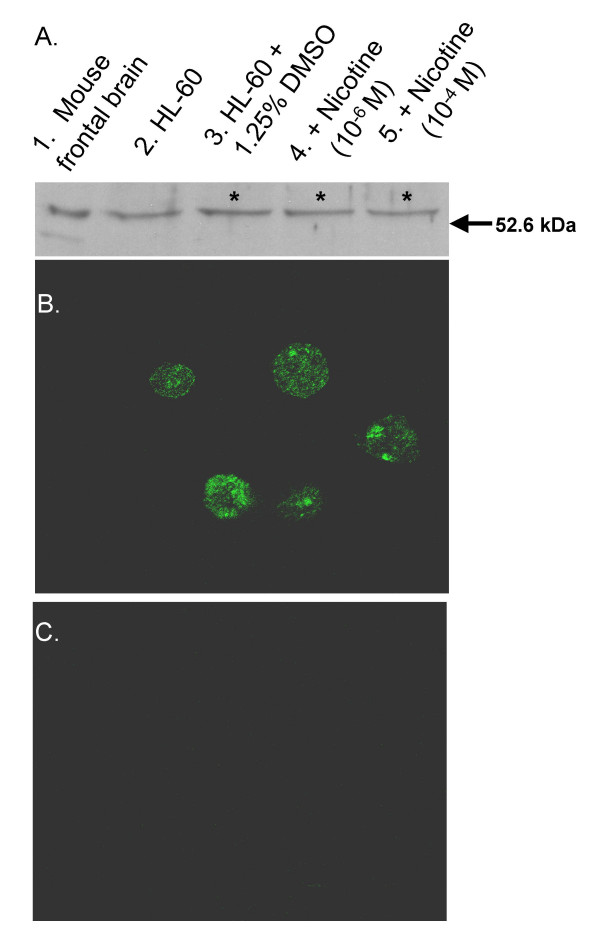
Figure 1.
Promyelocytic and DMSO-differentiated HL-60 cells express α7-acetylcholine nicotinic receptors. (A) α7 nAChR Western blot: Lane 1. Mouse frontal brain extract (10 μg; positive control); Lane 2. Lysate (40 μg) of promyelocytic HL-60 cells; Lanes 3 to 5. Lysate (40 μg) of five day, DMSO-induced HL-60 cells without nicotine, or with 10-6 M nicotine, or with 10-4 M nicotine treatment, respectively. α7 nAChR-specific antibodies (Santa Cruz SC-5544) bound to protein bands exhibiting a relative molecular mass of 55.0 KDa in all lanes. Densitometric analysis (data not shown) revealed that α7 nAChR-specific band intensities were significantly increased in the DMSO-treated samples, relative to promyelocytic cells (n = 5, *p < 0.05), but that this differentiation-associated increase α7 nAChR protein level was not influenced by nicotine exposure during differentiation in any statistically significant manner. (B) α7 nAChR Immunofluorescence staining (×1000): Fixed five-day, DMSO-induced HL-60 cells were incubated with a polyclonal rabbit anti-α7 antibody (Santa Cruz SC-5544) before staining with a FITC-conjugated anti-rabbit antibody (Santa Cruz SC-2253). α7 nAChR-specific staining was evenly distributed across the cells. A similar cellular distribution of α7 nAChR was observed in promyelocytic cells (data not shown). (C) Negative control for α7 nAChR Immunofluorescence staining (×1000).