Abstract
Telomeres are DNA–protein complexes at the ends of chromosomes that control genomic integrity but appear to become shorter with age and stress. To test whether stress causes telomere attrition, we exposed the offspring of wild-caught house mice (Mus musculus) to stressful conditions and examined the changes in telomere length over six months. We found that females exposed to males and reproductive stress (either with or without crowding) had significantly shorter telomeres than controls, and males exposed to crowding stress had shorter telomeres than males that were not crowded. Our results indicate that stress alters telomere dynamics, causing attrition and hindering restoration, and these effects are sex dependent. Telomeres may thus provide a biomarker for assessing an individual's cumulative exposure or ability to cope with stressful conditions.
Keywords: life history, senescence, ageing, oxidative stress, telomeres, mammals
1. Introduction
Reproduction has benefits but it also has costs due to reducing parental longevity and offspring quality (Roff 1992; Penn & Smith 2007); however, the proximate mechanisms underlying such life-history trade-offs are unclear (Zera & Harshman 2001). One important mechanistic link is oxidative stress, which occurs when the production of ‘free radicals’ from metabolic activities exceeds the capacity of antioxidant defences and damages DNA and repair mechanisms (Finkel & Holbrook 2000). Oxidative stress is a cost of growth and reproduction and contributes to ageing (Kirkwood & Austad 2000). Interestingly, there is increasing evidence that oxidative stress damages telomeres, the complex DNA–protein caps of eukaryotic chromosomes that function to maintain genome integrity (von Zglinicki 2002).
In vitro studies show that telomeres progressively shorten at each cell division until cell division ceases, and telomere attrition is therefore suspected to play a key role in senescence (Blackburn 2005). Many cross-sectional studies find an inverse correlation between age and telomere length (Monaghan & Haussmann 2006), but there is much variation in telomere length among age-matched individuals, indicating that factors other than chronological age affect telomere shortening. In vitro studies also show that oxidative stress causes telomere attrition due to direct damage or impairment of telomerase, an enzyme that functions to restore telomere shortening in vivo (von Zglinicki 2002). A recent study found that women under stress from rearing chronically ill children have shorter telomere lengths than controls, and as short as mothers 9–17 years older than themselves (Epel et al. 2004). This finding suggests that chronic stress causes telomere attrition, and that telomeres potentially provide a biomarker for cumulative exposure to oxidative stress. This stress hypothesis needs to be tested, however, with longitudinal studies and experimental manipulations with other species.
Our goal was to experimentally test whether chronic stress, either from reproduction or crowding, influences telomere dynamics in house mice (Mus musculus). We used the offspring of wild-trapped house mice, because laboratory mice do not provide a good model due to their unusually long telomeres (Hemann & Greider 2000). We manipulated reproduction and crowding, as these are ecologically relevant stressors that probably cause oxidative stress (Alonso-Alvarez et al. 2004; Wiersma et al. 2004; Miyashita et al. 2006), and examined the changes in telomere length over six months. Our results indicate that chronic stress affects telomere dynamics by causing attrition and hindering repair.
2. Material and methods
We used juvenile mice that were the offspring of wild-caught mice in Austria. We provided bedding, wood wool, tissues and cardboard tubes as nesting materials. Each experimental cage consisted of three standard (type II) mouse cages connected with plastic tubes to increase space (0.115 m2). Water and food (Altromin rodent diet 1324) were available ad libitum. The animals were kept under 12 : 12 h light/dark cycle with an ambient temperature of 23±3°C and relative humidity of 65±10%. The experiment was conducted from March to September 2005, and the procedures were approved by the Austrian Ministry of Education, Science and Cultures' Animal Care and Use Committee.
At four weeks of age, we assigned the animals to one of the three experimental groups. We housed females in large cages together with (i) one male and removed all offspring at 21 days of age to reduce interbirth intervals (reproduction treatment, REP), (ii) one male and we left female offspring to increase density (crowding treatment, CRD), or (iii) no males but one sister to avoid isolation stress (control, CTRL). We used the mean of two control females for our statistical analyses. To control for potential familial effects, we systematically assigned females from four-sister litters placing two in the control group and one each in treatment groups (each group was replicated 10 times). We did not include a similar control for males because males fight violently.
We collected blood to obtain DNA before the onset (before any births) and after termination of the experiment. To obtain DNA, we bled mice at 6, 14 and 16 weeks of age (75 μl each) and the blood was pooled (sample 1), stored in equal amounts of 50 mM EDTA and kept at −70°C for later DNA extraction. The experiment was terminated when the animals were six months old (mean age: 183.4±0.6 days). The animals were anaesthetized using ether, euthanized by rapid decapitation and blood was collected in 50 mM EDTA and frozen at −70°C (sample 2). We extracted DNA using a commercial kit (DNeasy Tissue Kit, Qiagen) and measured the mean telomere length of white blood cells using a real-time PCR method (Cawthon 2002) adapted for mice (Callicott & Womack 2006). A specially designed oligonucleotide primer set hybridizes to the TTAGGG and CCCTAA repeats and selectively amplifies telomeric DNA: longer telomeres lead to quantifiable acceleration of amplification. The amplification of telomeric DNA is compared to the amplification of a single-copy gene DNA of the same sample. We used MapK1 as the single-copy gene with the following primer sequences: Mapk1-F: 5′-GCT TAT GAT AAT CTC AAC AAA GTT CG-3′ and Mapk1-R: 5′-GAT GTT CTC ATG TCT GAA GCG-3′. All the samples were compared to one reference standard (relative telomere measurements), and the value of sample 1 (before treatment) was then subtracted from the value of sample 2 (after treatment). Negative values indicate telomeric attrition and positive values a gain. The procedure was carried out on an ABI (Applied Biosystems International) 7300 real-time PCR machine.
Before conducting statistical tests (SPSS 12.01), we confirmed that all assumptions were met. Since we had a priori predictions for the direction of telomere changes over time (attrition) and among groups (CTRL<(REP, CRD)), we used isotonic regression to test for among-group differences (Gaines & Rice 1990), directed Dunnett's pairwise multiple t-tests for females and a directed t-test for male differences (Rice & Gaines 1994). To test whether telomeres changed length over time, we compared the mean values with the 0 reference value by using one-sample t-tests. We predicted telomere attrition over time (mean telomere length change<0) and used directed tests. We obtained the critical values for each directed test from the p values of the corresponding one-tailed test, by using γ/α=0.8 as a pragmatic conventional value (Rice & Gaines 1994). Using two-sided tests does not change the interpretation of the results, except that the observed increase for telomeres in males of reproduction group becomes significant. Averages are shown as mean±s.e.m. Sample sizes between the experimental groups differ because two females and two males of the reproduction treatment and one female of the crowding treatment died during the experiment.
3. Results
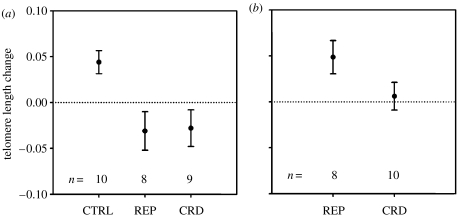
Females in the reproduction treatment weaned more offspring (25±4 total offspring) than the crowding (15±1) or control (0) groups. Females in the crowding treatment eventually ceased reproducing (final number of adults per cage: 9±1), verifying the stressfulness of these conditions. We found that females showed significantly different telomere changes among the three groups (isotonic regression: E32=0.29, p=0.007). Both the reproduction and crowding treatment groups had a significant reduction in telomere length compared with controls (figure 1a, Dunnett's t-test: p=0.01 for both), and there was no difference between the treatments (Tukey HSD: p=0.99). When pooled, the two stress treatments were significantly shorter than controls (t=2.86, p=0.002). The differences between the treatment and control groups were partly due to the shortening of telomeres of females in the treatment groups (t=−1.87, p=0.05 for pooled data), and also an unexpected increase of the telomeres over time in the controls (t=3.52, p=0.02). The telomere lengths of females in the reproduction treatment were significantly shorter than males in this treatment (figure 1, t=2.62, p=0.02).
Figure 1.
Mean change in telomere length (±s.e.m.) for (a) females and (b) males. The treatments include reproduction (REP), reproduction and crowding (CRD) or controls (CTRL). Negative values indicate telomere shortening, whereas positive values indicate a gain.
Unlike females, the males showed differences between the two treatment groups, such that the males exposed to crowding had shorter telomeres than those in the reproduction treatment (figure 1b, t=1.83, p=0.05). The telomeres of males in the crowding treatment did not change over time (t=0.40, p=1.00), whereas those in the reproduction treatment showed an unexpected tendency to increase (t=2.69, p=0.08; figure 1a,b), similar to control females.
4. Discussion
Females in both the reproduction and crowding treatments had a significant reduction in telomere length compared with controls. This difference was due to telomere attrition in the treatments and also an unexpected increase in the telomeres in the control females. Telomere lengthening with age has been observed in some cross-sectional studies (Hall et al. 2004), and longitudinal studies have also found telomere lengthening for some individuals (Gardner et al. 2005; Martin-Ruiz et al. 2005). The dynamics of telomeres of house mice have never been investigated before, to our knowledge, and it could be that telomeres increase through restoration when stress is minimized, which may be more probable in a laboratory setting (e.g. telomerase may continue to function and restore telomere length with low oxidative stress). An important caveat is that, like most studies on telomere dynamics, we cannot exclude the possibility that changes in telomeres were due to shifts in the composition of cell subtypes over time.
Exposing females to males resulted in telomere attrition both with and without crowding, which means that reproductive stress is sufficient to explain our results. Crowded females produced fewer offspring, and eventually ceased reproducing, but we cannot rule out potential stress effects from reproduction. Thus, the effect of crowding on females is unclear. Also, the source of stress in both treatments may have been sexually aggressive males rather than elevated reproduction per se (male mice are often sexually coercive). Socio-sexual aggression may be a normal component of female's costs of reproduction, though it should be possible to differentiate effects from sexual coercion versus reproduction using sterile males or artificial fertilization.
We also found that the telomeres of the males, unlike females, showed a difference between the reproduction and crowding treatments. This result was probably due to crowding, because the only difference between these treatments was crowding, and the reproduction treatment was probably not particularly stressful for males (males provide no parental care). Interestingly, this difference between the treatments was due to an unexpected telomeric increase for males in the reproduction group rather than a decline in the crowding group (this increase is not significant using a directed test, as we predicted a decrease over time, though it is significant if one applies a two-sided test). Thus, crowding did not cause telomere attrition for males, but it apparently interfered with telomere restoration that occurred when they were not crowded. The telomeric increase for males in the reproduction was opposite that of females in this treatment, and was comparable to the control (non-stressed) females. These results suggest that crowding stress hinders the restoration of telomeres.
In summary, our results support the hypothesis that stress affects telomere dynamics and the idea that telomere length might indicate an individual's exposure or resistance to oxidative stress (Epel et al. 2004). Since males and females responded differently to reproductive and crowding stress, our results might also help explain sex differences in telomere length and why these differences develop over time (Cherif et al. 2003). Finally, reproduction reduces longevity in mice and other species (Russell 1964; Roff 1992), especially in females (Penn & Smith 2007), and our results suggest that telomeres might play a role in mediating such life-history trade-offs.
Acknowledgments
We are grateful to R. M. Cawthon for advice, A. Joachim for use of her qPCR facilities, R. M. Sapolsky and S. M. Zala for comments on our manuscript and H. Wolfger and M. Zöttl for assistance.
References
- Alonso-Alvarez C, Bertrand S, Devevey G, Prost J, Faivre B, Sorci G. Increased susceptibility to oxidative stress as a proximate cost of reproduction. Ecol. Lett. 2004;7:363–368. doi:10.1111/j.1461-0248.2004.00594.x [Google Scholar]
- Blackburn E.H. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. doi:10.1016/j.febslet.2004.11.036 [DOI] [PubMed] [Google Scholar]
- Callicott R.J, Womack J.E. Real-time PCR assay for measurement of mouse telomeres. Comp. Med. 2006;56:17–22. [PubMed] [Google Scholar]
- Cawthon R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:E47. doi: 10.1093/nar/30.10.e47. doi:10.1093/nar/30.10.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif H, Tarry J.L, Ozanne S.E, Hales C.N. Ageing and telomeres: a study into organ- and gender-specific telomere shortening. Nucleic Acids Res. 2003;31:1576–1583. doi: 10.1093/nar/gkg208. doi:10.1093/nar/gkg208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E.S, Blackburn E.H, Lin J, Dhabhar F.S, Adler N.E, Morrow J.D, Cawthon R.M. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA. 2004;101:17 312–17 315. doi: 10.1073/pnas.0407162101. doi:10.1073/pnas.0407162101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook N.J. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. doi:10.1038/35041687 [DOI] [PubMed] [Google Scholar]
- Gaines S.D, Rice W.R. Analysis of biological data when there are ordered expectations. Am. Nat. 1990;135:310–317. doi:10.1086/285047 [Google Scholar]
- Gardner J.P, Li S, Srinivasan S.R, Chen W, Kimura M, Lu X, Berenson G.S, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. doi:10.1161/01.CIR.0000163550.70487.0B [DOI] [PubMed] [Google Scholar]
- Hall M.E, Nasir L, Daunt F, Gault E.A, Croxall J.P, Wanless S, Monaghan P. Telomere loss in relation to age and early environment in long-lived birds. Proc. R. Soc. B. 2004;271:1571–1576. doi: 10.1098/rspb.2004.2768. doi:10.1098/rspb.2004.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemann M.T, Greider C.W. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28:4474–4478. doi: 10.1093/nar/28.22.4474. doi:10.1093/nar/28.22.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood T.B.L, Austad S.N. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. doi:10.1038/35041682 [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz C.M, Gussekloo J, van Heemst D, von Zglinicki T, Westendorp R.G. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. doi:10.1111/j.1474-9726.2005.00171.x [DOI] [PubMed] [Google Scholar]
- Miyashita T, Yamaguchi T, Motoyama K, Unno K, Nakano Y, Shimoi K. Social stress increases biopyrrins, oxidative metabolites of bilirubin, in mouse urine. Biochem. Biophys. Res. Commun. 2006;349:775–780. doi: 10.1016/j.bbrc.2006.08.098. doi:10.1016/j.bbrc.2006.08.098 [DOI] [PubMed] [Google Scholar]
- Monaghan P, Haussmann M.F. Do telomere dynamics link lifestyle and lifespan? Trends Ecol. Evol. 2006;21:47–53. doi: 10.1016/j.tree.2005.11.007. doi:10.1016/j.tree.2005.11.007 [DOI] [PubMed] [Google Scholar]
- Penn D.J, Smith K.R. Differential fitness costs of reproduction between the sexes. Proc. Natl Acad. Sci. USA. 2007;104:553–558. doi: 10.1073/pnas.0609301103. doi:10.1073/pnas.0609301103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice W.R, Gaines S.D. ’Heads I win, tails you lose’: testing directional alternative hypotheses in ecological and evolutionary research. Trend Ecol. Evol. 1994;9:235–237. doi: 10.1016/0169-5347(94)90258-5. doi:10.1016/0169-5347(94)90258-5 [DOI] [PubMed] [Google Scholar]
- Roff D.A. Chapman & Hall; New York, NY: 1992. The evolution of life histories: theory and analysis. [Google Scholar]
- Russell E.S. Lifespan and aging patterns. In: Green E.L, editor. Biology of the laboratory mouse. Dover Publications; New York, NY: 1964. pp. 685–692. [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem. Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. doi:10.1016/S0968-0004(02)02110-2 [DOI] [PubMed] [Google Scholar]
- Wiersma P, Selman C, Speakman J.R, Verhulst S. Birds sacrifice oxidative protection for reproduction. Proc. R. Soc. B. 2004;271:S360–S363. doi: 10.1098/rsbl.2004.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zera A.J, Harshman L.G. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 2001;32:95–126. doi:10.1146/annurev.ecolsys.32.081501.114006 [Google Scholar]