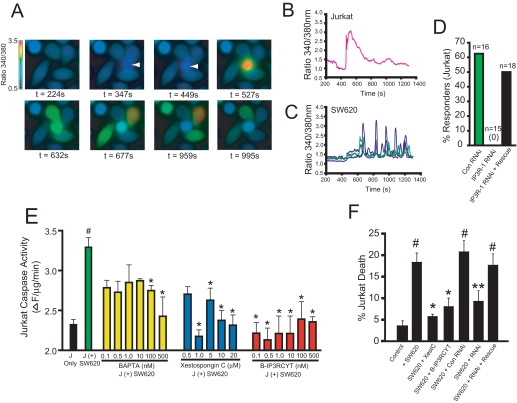
FIGURE 1.
Calcium release from IP3R is required for SW620-mediated killing of Jurkat cells. A, Fura-2 calcium ratio imaging of SW620/Jurkat co-cultures. The first panel shows only adherent SW620 cells. At a time of 347 s, a single Jurkat cell (second panel; indicated by an arrow), comes into contact with four SW620 cells, which leads to a rise in intracellular calcium. B, calcium response in the Jurkat cell shown in A. C, calcium responses of the four SW620 cells shown in A. Qualitatively similar results were observed in all trials in which a Jurkat cell contacted at least one SW620 cell (data not shown). D, histogram of the percentage of Jurkat cells, which released calcium in response to binding SW620 cells after transfection with control RNAi (Con RNAi), IP3R-1 RNAi, or co-transfection with IP3R-1 RNAi and the cDNA encoding the rat IP3R-1. The total number of transfected cells that contacted an SW620 cell is indicated. No cells expressing IP3R-1 RNAi responded (0/15). E, caspase-3-like activity (DEVDase activity) in Jurkat cells co-cultured with SW620 cells or cultured alone (black bar). Green, no pretreatment before co-culture for 24 h with SW620 cells. Yellow, Jurkat cells were preincubated with the indicated concentrations of BAPTA-AM before co-culture. Blue, Jurkat cells were preincubated with the indicated concentrations of xestospongin C. Red, Jurkat cells were preincubated with the indicated concentrations of B-IP3RCYT. See “Experimental Procedures” for details. J = Jurkat. F, cell death in Jurkat cells prior to (Control) or 24 h after co-culture with SW620 cells (+SW620). The effects of 1 μm XestC and 10 nm B-IP3RCYT were tested by preincubation prior to co-culture. The indicated RNAis were transfected 24 h before co-culture as described elsewhere (12). All data represent the mean ± S.E. # indicates p < 0.05 relative to control (no incubation with SW620 cells). * indicates p < 0.05 relative to co-culture with SW620 cells. ** indicates p < 0.05 relative to co-culture with SW620 + control RNAi.