FIGURE 2.
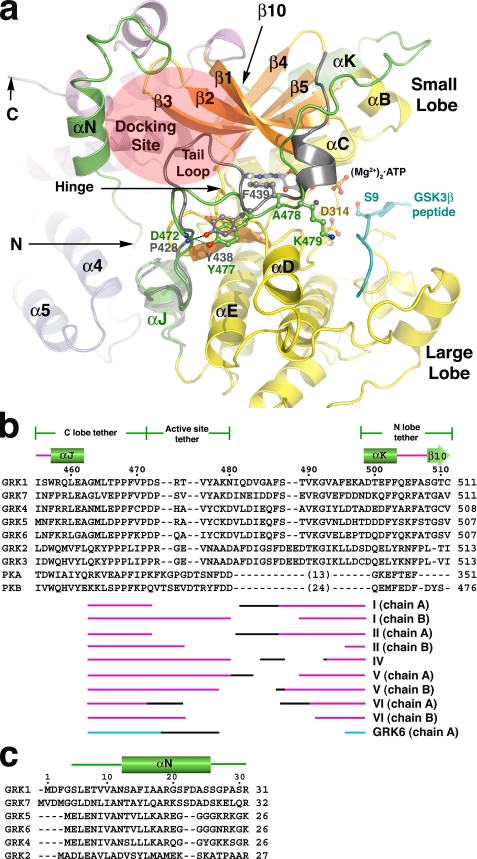
The N terminus and kinase C-terminal extension of GRK1. a, the active site regions of GRK1 and PKB. For this comparison, the coordinates of the various GRK1 crystal forms were merged to generate a composite structure of GRK1 that spans residues 5-533 (of 558), and the large lobe was rotated to generate the expected closed, active state. The kinase C-terminal extension of PKB is gray, and the GSK3β peptide bound to PKB is cyan. The tail-loop of GRKs is four residues shorter than those of either PKB or PKA, resulting in formation of a shallow canyon with the hinge of the kinase domain at the bottom. This region forms a putative receptor-docking site (transparent ellipse). b, structural alignment of the kinase C-terminal extension of GRKs with PKA and PKB. The region immediately following the active site tether is structurally divergent among GRKs, PKA, and PKB, and thus no alignment is attempted. The numbers above the alignment refer to the sequence of bovine GRK1. The different segments of the C-terminal extension that were observed in each of our crystal forms are indicated below the alignment, with the black regions corresponding to structurally heterogeneous portions in each structure that appear influenced by crystal contacts. The accession codes for the protein sequences are: GRK1, P28327; GRK2, NP_777135.1; GRK3, P26818; GRK4, AAI17321.1; GRK5, P43249; GRK6, P43250; GRK7, NP_776757.1; PKA, 1L3R; PKB, 1O6L. c, structural alignment of GRK N-terminal regions.