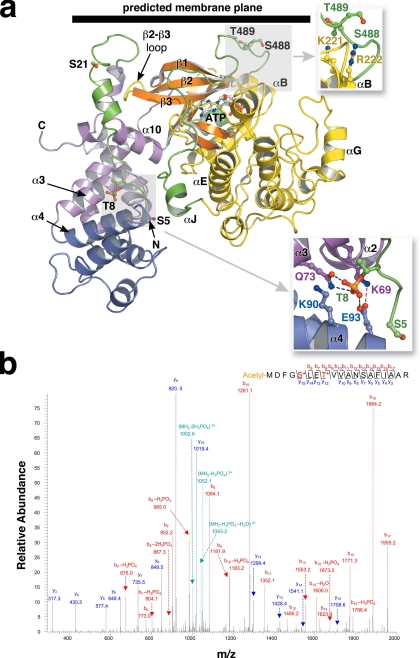
FIGURE 3.
The phosphorylation sites of GRK1. a, the RH-kinase core of GRK1. The structure corresponds to that of crystal form I (with composite C-terminal extension; see Fig. 2). The Ser5, Thr8, Ser21, Ser488, and Thr489 phosphorylation sites are drawn as stick models. The expected position of the membrane plane is indicated. Top inset, the Ser488 and Thr489 phosphorylation sites correspond to the AGC kinase turn motif. Bottom inset, interaction of Thr(P)8 with the RH domain. Gln73 and Glu93 form direct hydrogen bonds, whereas Lys69 and Lys90 complement the charge of the phosphate moiety. These crystals grew at pH 4.35, and so either Glu93 or the phosphate group could be protonated. b, tandem mass spectrometry spectra of phosphopeptides from GRK1535-His6 (Pool A, pretreated with 4 mm ATP and 2 mm MgCl2). Both Ser5 and Thr8 sites were identified in a single peptide. The Ser5 site was also readily observed in endogenous GRK1, as were the previously observed phosphorylation sites at Ser21, Ser488, and Thr489 (supplemental Fig. S7).