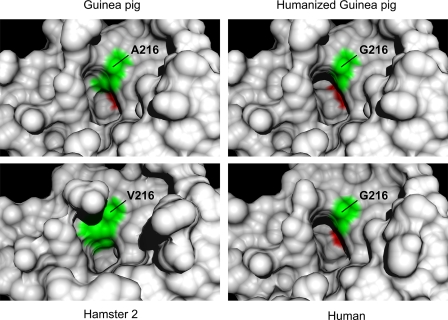
FIGURE 6.
Models of P1 side chain binding pocket. The panels show corresponding regions of wild type guinea pig, humanized A216G guinea pig, human, and hamster 2 chymases. The human and hamster models are based on crystal-derived structures of Protein Data Bank code 1pjp and 2rdl, respectively. The guinea pig structures are homology models. Specificity triad residues 216 and 226 (chymotrypsinogen numbering) are green and red, respectively. Note that the slotlike crevice accommodating the aromatic P1 side chain in the human enzyme is partly filled in by the Ala216 side chain of wild type guinea pig chymase but is largely restored in the humanized A216G mutant. By comparison, the bulky side chain of Val216 in hamster chymase 2 markedly reduces the volume available for occupancy by a P1 side chain.