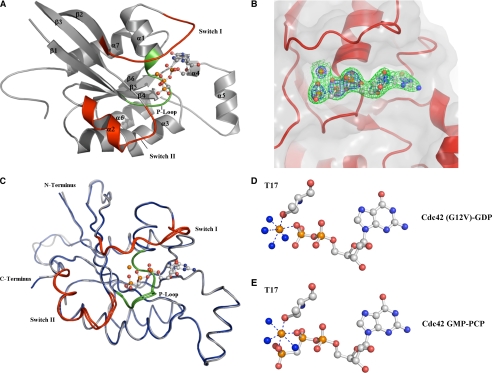
FIGURE 2.
X-ray crystal structure of GMP-PCP bound Cdc42 at 2.4 Å. A, overall fold of GMP-PCP-bound Cdc42 is a classic G domain encompassing six β-sheets and five α-helices with a short two-helical insertion after the β-strand 5 known as the Rho insert region. The α-helix 4 is a short 310 helix. Switch I and II are colored in red encompassing residues 30-37 and 60-70, respectively. P-loop residues, which are important for binding the phosphates, are colored green. B, electron density around the GMP-PCP is contoured at 1.5σ (green) and 4σ (blue) from a 2Fo - Fc map. Clear density is present for the γ-phosphate even at 4σ. C, structural alignment of the signaling-active Cdc42-GMP-PCP complex (gray) with the signaling-inactive Cdc42 (G12V)-GDP (blue). Switch regions and the P-loop are colored red and green, respectively. There is an overall r.m.s.d. of 0.75 Å for all C-α atoms between the two structures. D and E, close-up views of the Mg2+ coordination between the Cdc42 (G12V)-GDP and Cdc42-GMP-PCP structures, respectively. Phosphates and magnesium are shown in orange, and water and nitrogen molecules are colored blue. Threonine 35 does not coordinate to the γ-phosphate in the Cdc42-GMP-PCP structure as it does in other signaling active structures. It is replaced by a water molecule in the Cdc42 structures, whereas all other contacts are conserved. Figs. 2 and 3 were created with PyMOL (50).