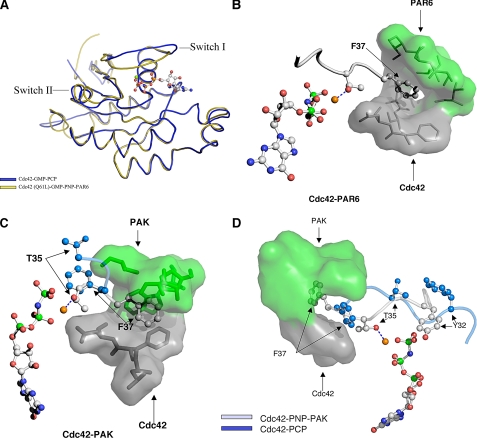
FIGURE 6.
Effector proteins stabilize Switch I in an “active” conformation. A, comparisons of the overall fold of GMP-PCP-bound Cdc42 versus GMP-PNP-bound Cdc42(Q61L) complexed to Par6 (PDB ID 1NF3). B, Switch I residues Thr-35 and Phe-37 for Cdc42(Q61L)-GMP-PNP bound to the nonconventional Cdc42/Rac-interactive-binding domain of Par6 are shown in gray. Par6 residues (in green) form the lid to a hydrophobic pocket for Phe-37, with the bottom of the pocket being contributed by residues from Cdc42 (gray). Phe-37 acts as a fulcrum lever to flip Thr-35 into position to coordinate the Mg2+. C, same view of Cdc42(Q61L)-GMP-PNP bound to the CRIB domain of Pak1 based on the NMR structure for this complex (PDB ID 1E0A). Phe-37 is embedded in a hydrophobic pocket created by both Pak1 (green) and Cdc42 (gray). An overlay of Thr-35 and Phe-37 from Cdc42-GMP-PCP is displayed in blue to illustrate the lever action and stabilization of Phe-37 by Pak. D, overlay of Switch I from the NMR structure for the Cdc42-GMP-PNP-Pak1 complex (PDB ID 1E0A) and the x-ray structure for the Cdc42-GMP-PCP complex. Notice that in the Pak1 complex, Switch I residues from Cdc42 are rotated 180° from their position in the Cdc42-GMP-PCP structure and are stabilized by the interaction of Phe-37 with the effector protein.