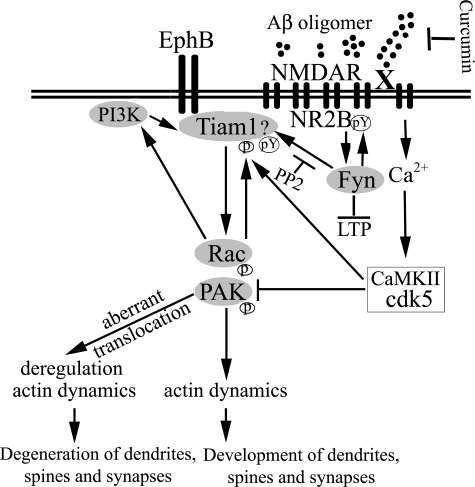
FIGURE 8.
Schematic mechanism of Aβ oligomer-mediated aberrant PAK activation and translocation in Alzheimer disease pathogenesis. Both Ephrin B and NMDA receptors are rapidly down-regulated by Aβ oligomers (28) and also activate a Tiam1> Rac > PAK pathway that regulates dendritic spine stability and formation through control of actin dynamics, including suppression of the actin-severing protein cofilin reported to mediate oligomer-induced spine loss (1, 2). Tiam1 activation is also required for acute Aβ aggregate-induced Rac activation and translocation (45) that we show accompanies PAK translocation. Tiam1 activation can be achieved by phosphorylation from elevated calcium acting through CaMKII, but PAK is eventually inhibited by calcium-induced calpain activation of Cdk5 (50). PAK could also be activated through direct interaction with candidate Aβ receptors (X), for example, the amyloid precursor protein (47) or indirectly via Src/Fyn activation of Tiam1. Although the Aβ receptors are not clearly established, Aβ-mediated in vivo synaptotoxicity and in vitro LTP deficits are both Fyn kinase-(32, 33) and NMDA receptor-dependent (1, 2). The Src family kinase Fyn is also physically associated with at least two candidate Aβ receptors (X), notably integrin receptors (51, 52) and α7-nicotinic receptors (53), suggesting they could activate Fyn to mediate deranged downstream Rac/PAK translocation and signaling caused by Aβ oligomers, which we find blocked by the anti-oligomer drug curcumin. This hypothetical scheme is supported by our data showing that the Src/Fyn inhibitor PP2 blocks the Rac/PAK translocation and loss of dendritic spine F-actin.