Abstract
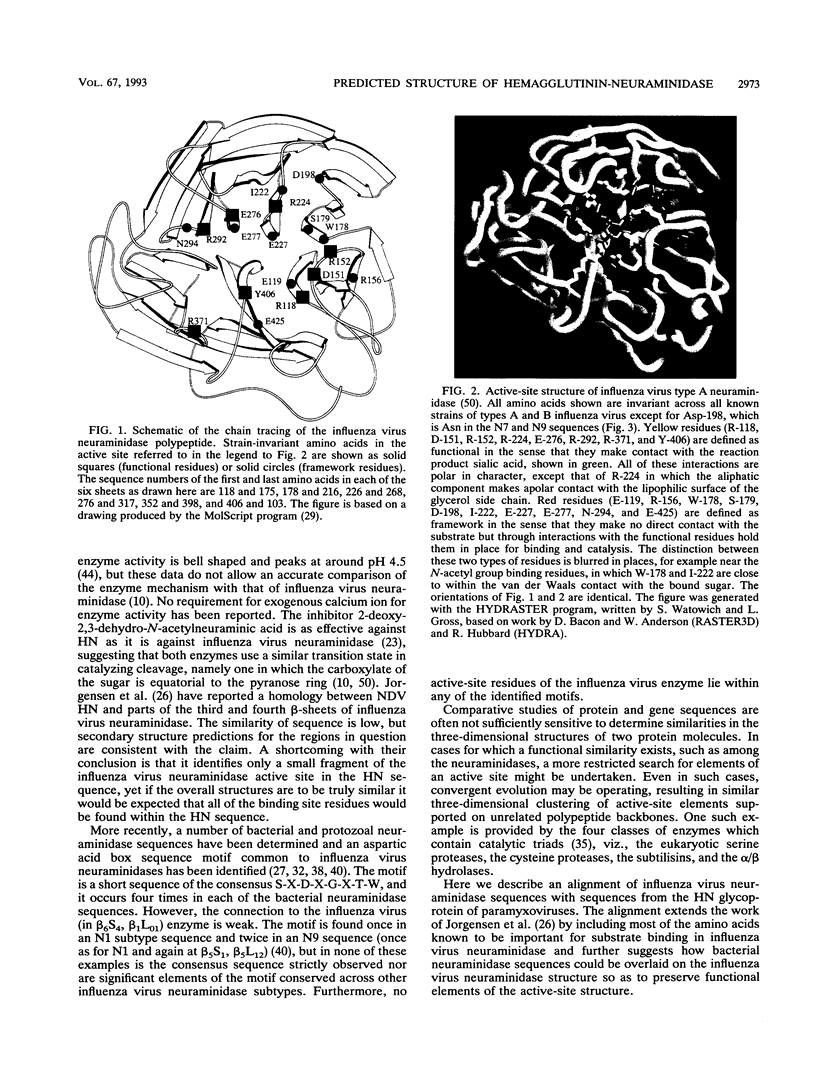
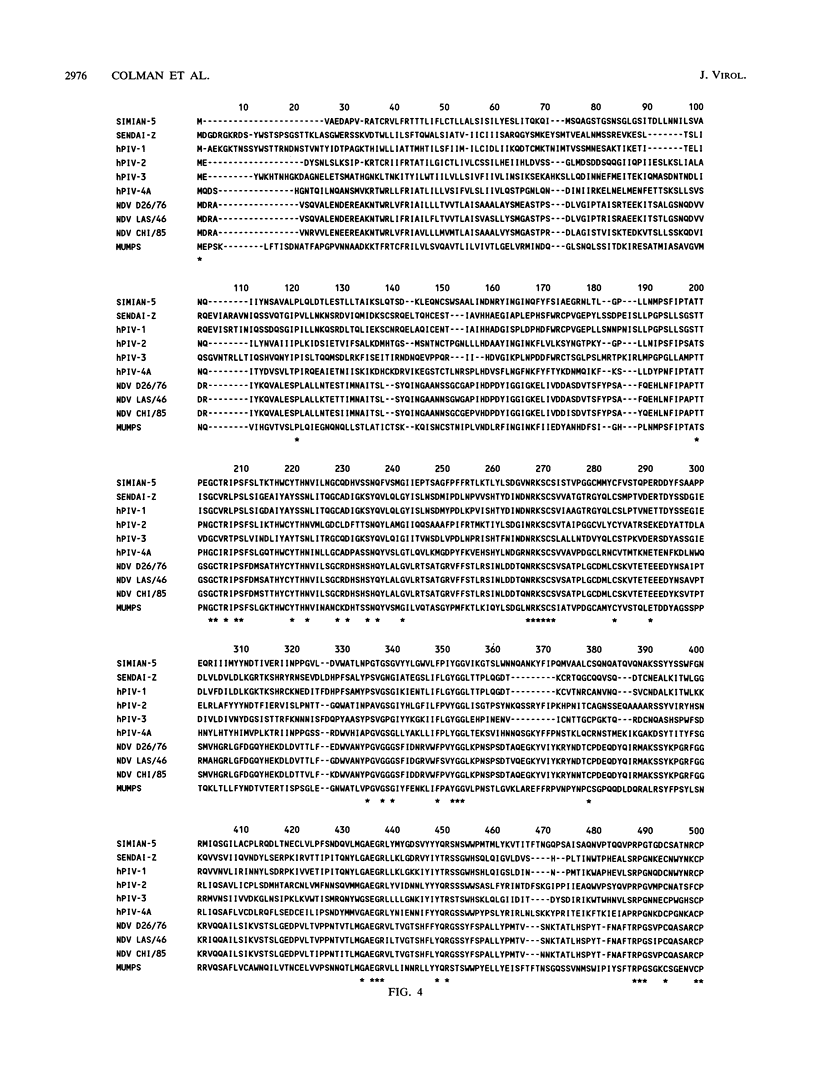
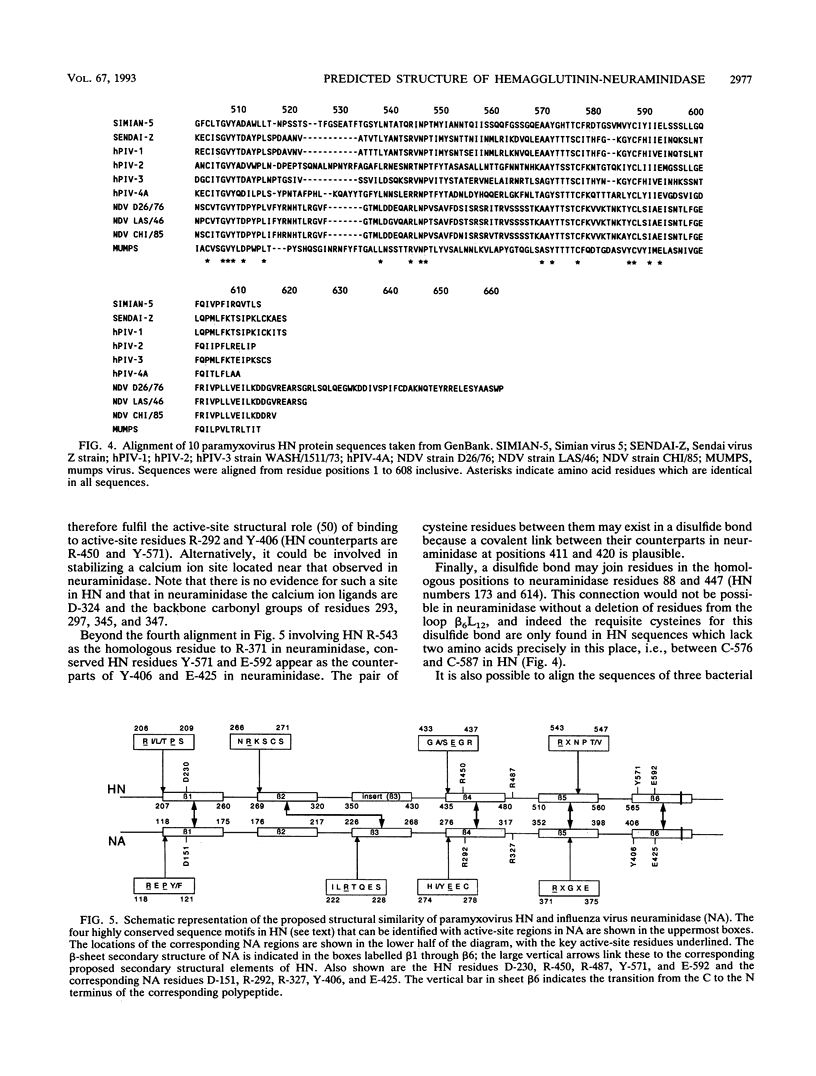
A model is proposed for the three-dimensional structure of the paramyxovirus hemagglutinin-neuraminidase (HN) protein. The model is broadly similar to the structure of the influenza virus neuraminidase and is based on the identification of invariant amino acids among HN sequences which have counterparts in the enzyme-active center of influenza virus neuraminidase. The influenza virus enzyme-active site is constructed from strain-invariant functional and framework residues, but in this model of HN, it is primarily the functional residues, i.e., those that make direct contact with the substrate sialic acid, which have identical counterparts in neuraminidase. The framework residues of the active site are different in HN and in neuraminidase and appear to be less strictly conserved within HN sequences than within neuraminidase sequences.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Air G. M., Laver W. G., Luo M., Stray S. J., Legrone G., Webster R. G. Antigenic, sequence, and crystal variation in influenza B neuraminidase. Virology. 1990 Aug;177(2):578–587. doi: 10.1016/0042-6822(90)90523-t. [DOI] [PubMed] [Google Scholar]
- Air G. M., Ritchie L. R., Laver W. G., Colman P. M. Gene and protein sequence of an influenza neuraminidase with hemagglutinin activity. Virology. 1985 Aug;145(1):117–122. doi: 10.1016/0042-6822(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Baker A. T., Varghese J. N., Laver W. G., Air G. M., Colman P. M. Three-dimensional structure of neuraminidase of subtype N9 from an avian influenza virus. Proteins. 1987;2(2):111–117. doi: 10.1002/prot.340020205. [DOI] [PubMed] [Google Scholar]
- Bando H., Kondo K., Kawano M., Komada H., Tsurudome M., Nishio M., Ito Y. Molecular cloning and sequence analysis of human parainfluenza type 4A virus HN gene: its irregularities on structure and activities. Virology. 1990 Mar;175(1):307–312. doi: 10.1016/0042-6822(90)90213-b. [DOI] [PubMed] [Google Scholar]
- Basak S., Tomana M., Compans R. W. Sialic acid is incorporated into influenza hemagglutinin glycoproteins in the absence of viral neuraminidase. Virus Res. 1985 Feb;2(1):61–68. doi: 10.1016/0168-1702(85)90060-7. [DOI] [PubMed] [Google Scholar]
- Burmeister W. P., Ruigrok R. W., Cusack S. The 2.2 A resolution crystal structure of influenza B neuraminidase and its complex with sialic acid. EMBO J. 1992 Jan;11(1):49–56. doi: 10.1002/j.1460-2075.1992.tb05026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A. K., Pegg M. S., Taylor N. R., von Itzstein M. Evidence for a sialosyl cation transition-state complex in the reaction of sialidase from influenza virus. Eur J Biochem. 1992 Jul 1;207(1):335–343. doi: 10.1111/j.1432-1033.1992.tb17055.x. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Varghese J. N., Laver W. G. Structure of the catalytic and antigenic sites in influenza virus neuraminidase. Nature. 1983 May 5;303(5912):41–44. doi: 10.1038/303041a0. [DOI] [PubMed] [Google Scholar]
- Curran M. D., Clarke D. K., Rima B. K. The nucleotide sequence of the gene encoding the attachment protein H of canine distemper virus. J Gen Virol. 1991 Feb;72(Pt 2):443–447. doi: 10.1099/0022-1317-72-2-443. [DOI] [PubMed] [Google Scholar]
- Dale B., Brown R., Miller J., White R. T., Air G. M., Cordell B. Nucleotide and deduced amino acid sequence of the influenza neuraminidase genes of two equine serotypes. Virology. 1986 Dec;155(2):460–468. doi: 10.1016/0042-6822(86)90207-2. [DOI] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase; its substrate and mode of action. Adv Enzymol Relat Subj Biochem. 1958;20:135–146. doi: 10.1002/9780470122655.ch5. [DOI] [PubMed] [Google Scholar]
- Galen J. E., Ketley J. M., Fasano A., Richardson S. H., Wasserman S. S., Kaper J. B. Role of Vibrio cholerae neuraminidase in the function of cholera toxin. Infect Immun. 1992 Feb;60(2):406–415. doi: 10.1128/iai.60.2.406-415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman W. L., Gill D. S., Scroggs R. A., Portner A. The hemagglutinin-neuraminidase glycoproteins of human parainfluenza virus type 1 and Sendai virus have high structure-function similarity with limited antigenic cross-reactivity. Virology. 1990 Mar;175(1):211–221. doi: 10.1016/0042-6822(90)90201-2. [DOI] [PubMed] [Google Scholar]
- Griffin J. A., Basak S., Compans R. W. Effects of hexose starvation and the role of sialic acid in influenza virus release. Virology. 1983 Mar;125(2):324–334. doi: 10.1016/0042-6822(83)90205-2. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Ward C. W., Hudson P. J. Molecular cloning and analysis of the N5 neuraminidase subtype from an avian influenza virus. Virology. 1989 Mar;169(1):239–243. doi: 10.1016/0042-6822(89)90063-9. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Paterson R. G., Lamb R. A. Hemagglutinin-neuraminidase protein of the paramyxovirus simian virus 5: nucleotide sequence of the mRNA predicts an N-terminal membrane anchor. J Virol. 1985 Apr;54(1):1–6. doi: 10.1128/jvi.54.1.1-6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hoyer L. L., Hamilton A. C., Steenbergen S. M., Vimr E. R. Cloning, sequencing and distribution of the Salmonella typhimurium LT2 sialidase gene, nanH, provides evidence for interspecies gene transfer. Mol Microbiol. 1992 Apr;6(7):873–884. doi: 10.1111/j.1365-2958.1992.tb01538.x. [DOI] [PubMed] [Google Scholar]
- Iorio R. M., Syddall R. J., Glickman R. L., Riel A. M., Sheehan J. P., Bratt M. A. Identification of amino acid residues important to the neuraminidase activity of the HN glycoprotein of Newcastle disease virus. Virology. 1989 Nov;173(1):196–204. doi: 10.1016/0042-6822(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Jorgensen E. D., Collins P. L., Lomedico P. T. Cloning and nucleotide sequence of Newcastle disease virus hemagglutinin-neuraminidase mRNA: identification of a putative sialic acid binding site. Virology. 1987 Jan;156(1):12–24. doi: 10.1016/0042-6822(87)90431-4. [DOI] [PubMed] [Google Scholar]
- Kahn S., Colbert T. G., Wallace J. C., Hoagland N. A., Eisen H. The major 85-kDa surface antigen of the mammalian-stage forms of Trypanosoma cruzi is a family of sialidases. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4481–4485. doi: 10.1073/pnas.88.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz M. R., Air G. M., Laver W. G., Webster R. G. Sequence of the neuraminidase gene of influenza virus A/Tokyo/3/67 and previously uncharacterized monoclonal variants. Virology. 1984 May;135(1):257–265. doi: 10.1016/0042-6822(84)90135-1. [DOI] [PubMed] [Google Scholar]
- Lentz M. R., Webster R. G., Air G. M. Site-directed mutation of the active site of influenza neuraminidase and implications for the catalytic mechanism. Biochemistry. 1987 Aug 25;26(17):5351–5358. doi: 10.1021/bi00391a020. [DOI] [PubMed] [Google Scholar]
- Miura N., Nakatani Y., Ishiura M., Uchida T., Okada Y. Molecular cloning of a full-length cDNA encoding the hemagglutinin-neuraminidase glycoprotein of Sendai virus. FEBS Lett. 1985 Aug 19;188(1):112–116. doi: 10.1016/0014-5793(85)80885-1. [DOI] [PubMed] [Google Scholar]
- Ollis D. L., Cheah E., Cygler M., Dijkstra B., Frolow F., Franken S. M., Harel M., Remington S. J., Silman I., Schrag J. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- Palese P., Compans R. W. Inhibition of influenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA): mechanism of action. J Gen Virol. 1976 Oct;33(1):159–163. doi: 10.1099/0022-1317-33-1-159. [DOI] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E., Mejia J. S., Ortega-Barria E., Matzilevich D., Prioli R. P. The Trypanosoma cruzi neuraminidase contains sequences similar to bacterial neuraminidases, YWTD repeats of the low density lipoprotein receptor, and type III modules of fibronectin. J Exp Med. 1991 Jul 1;174(1):179–191. doi: 10.1084/jem.174.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Kaper J. B., Galen J., Lawrisuk L., Vimr E. R., Schauer R. Conserved sequences in bacterial and viral sialidases. Glycoconj J. 1989;6(3):349–353. doi: 10.1007/BF01047853. [DOI] [PubMed] [Google Scholar]
- Roggentin P., Rothe B., Lottspeich F., Schauer R. Cloning and sequencing of a Clostridium perfringens sialidase gene. FEBS Lett. 1988 Sep 26;238(1):31–34. doi: 10.1016/0014-5793(88)80219-9. [DOI] [PubMed] [Google Scholar]
- Rothe B., Roggentin P., Frank R., Blöcker H., Schauer R. Cloning, sequencing and expression of a sialidase gene from Clostridium sordellii G12. J Gen Microbiol. 1989 Nov;135(11):3087–3096. doi: 10.1099/00221287-135-11-3087. [DOI] [PubMed] [Google Scholar]
- Sakaguchi T., Toyoda T., Gotoh B., Inocencio N. M., Kuma K., Miyata T., Nagai Y. Newcastle disease virus evolution. I. Multiple lineages defined by sequence variability of the hemagglutinin-neuraminidase gene. Virology. 1989 Apr;169(2):260–272. doi: 10.1016/0042-6822(89)90151-7. [DOI] [PubMed] [Google Scholar]
- Scheid A., Caliguiri L. A., Compans R. W., Choppin P. W. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972 Dec;50(3):640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- Taylor G., Vimr E., Garman E., Laver G. Purification, crystallization and preliminary crystallographic study of neuraminidase from Vibrio cholerae and Salmonella typhimurium LT2. J Mol Biol. 1992 Aug 20;226(4):1287–1290. doi: 10.1016/0022-2836(92)91069-2. [DOI] [PubMed] [Google Scholar]
- Tulip W. R., Varghese J. N., Baker A. T., van Donkelaar A., Laver W. G., Webster R. G., Colman P. M. Refined atomic structures of N9 subtype influenza virus neuraminidase and escape mutants. J Mol Biol. 1991 Sep 20;221(2):487–497. doi: 10.1016/0022-2836(91)80069-7. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Colman P. M. Three-dimensional structure of the neuraminidase of influenza virus A/Tokyo/3/67 at 2.2 A resolution. J Mol Biol. 1991 Sep 20;221(2):473–486. doi: 10.1016/0022-2836(91)80068-6. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Laver W. G., Colman P. M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 A resolution. Nature. 1983 May 5;303(5912):35–40. doi: 10.1038/303035a0. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., McKimm-Breschkin J. L., Caldwell J. B., Kortt A. A., Colman P. M. The structure of the complex between influenza virus neuraminidase and sialic acid, the viral receptor. Proteins. 1992 Nov;14(3):327–332. doi: 10.1002/prot.340140302. [DOI] [PubMed] [Google Scholar]
- Varghese J. N., Webster R. G., Laver W. G., Colman P. M. Structure of an escape mutant of glycoprotein N2 neuraminidase of influenza virus A/Tokyo/3/67 at 3 A. J Mol Biol. 1988 Mar 5;200(1):201–203. doi: 10.1016/0022-2836(88)90344-0. [DOI] [PubMed] [Google Scholar]
- Warner T. G., Harris R., McDowell R., Vimr E. R. Photolabelling of Salmonella typhimurium LT2 sialidase. Identification of a peptide with a predicted structural similarity to the active sites of influenza-virus sialidases. Biochem J. 1992 Aug 1;285(Pt 3):957–964. doi: 10.1042/bj2850957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J. Identification of amino acids involved in the sialidase activity of the mumps virus hemagglutinin-neuraminadase protein. Virology. 1988 Nov;167(1):226–232. doi: 10.1016/0042-6822(88)90072-4. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Server A. C., Wolinsky J. S., Smith J. A., Goodman H. M. Sequence determination of the mumps virus HN gene. Virology. 1988 Jun;164(2):318–325. doi: 10.1016/0042-6822(88)90544-2. [DOI] [PubMed] [Google Scholar]
- van Wyke Coelingh K. L., Winter C. C., Murphy B. R. Nucleotide and deduced amino acid sequence of hemagglutinin-neuraminidase genes of human type 3 parainfluenza viruses isolated from 1957 to 1983. Virology. 1988 Jan;162(1):137–143. doi: 10.1016/0042-6822(88)90402-3. [DOI] [PubMed] [Google Scholar]