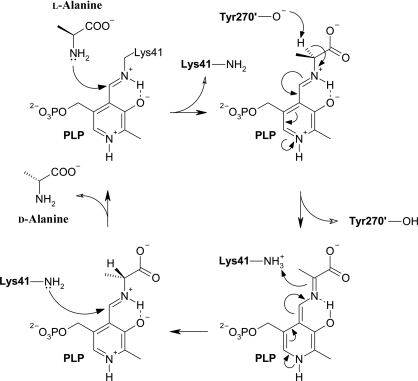
Figure 1.
The classical two-base mechanism of the reaction catalyzed by alanine racemase using pyridoxal 5′-phosphate as a cofactor. The reaction is shown in the direction of conversion from l-alanine to d-alanine, in which Tyr270′ abstracts a proton from l-alanine to form a planar intermediate and Lys41 donates a proton to form d-alanine. A more detailed mechanism avoiding the creation of a quinonoid intermediate has been proposed by Watanabe et al. (2002 ▶).