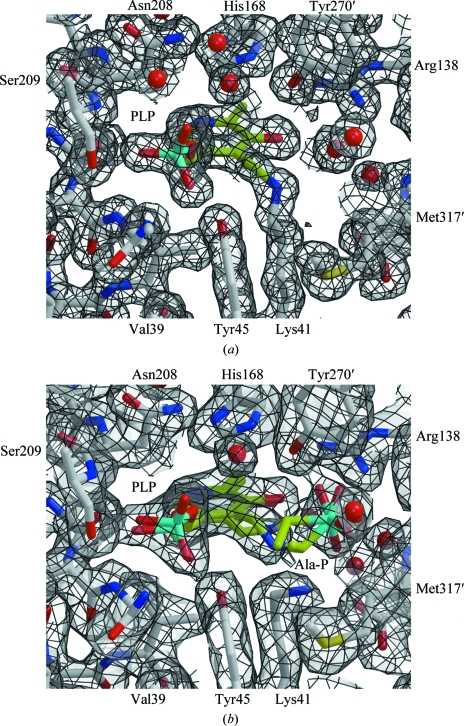
Figure 4.
The active site of B. anthracis alanine racemase. (a) The structure of the active site (atom-coloured ball-and-stick representation; carbon in grey) highlighting the PLP adduct (carbon in lime green) and showing 2F obs − F calc electron density contoured at 1.0σ (grey surface with black lines). Residues surrounding the active site are labelled, with the exceptions of Arg224–Ile227 and Tyr359, which form the back and front faces of the site as shown, respectively. The electron density shows the formation of the Schiff base between Lys41 and the PLP cofactor. (b) An equivalent view of the structure in the presence of inhibitor. The ball-and-stick models with lime green C atoms show the refined positions for a racemic mixture of PLP–l-Ala-P and PLP–d-Ala-P.