Figure 1.
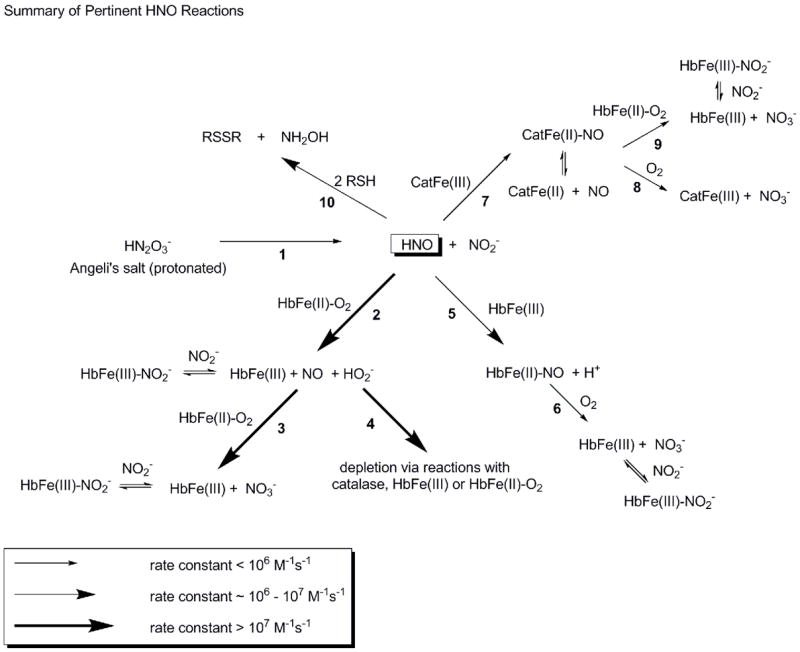
Schematic of reactions involving Angeli’s salt relevant to this study. Larger arrows indicate faster reactions as shown in the legend. Each reaction is numbered under the arrow. In reaction 1, AS decomposes to HNO and nitrite with a rate constant of about 6 × 10−4 s−1 in our studies, similar to that reported previously [84]. HNO reacts with oxyHb (reaction 2) with a rate constant of about 1 × 107 M−1s−1 (based on that reported for oxymyoglobin [85]), forming metHb, NO and peroxide. NO reacts with oxyHb to form metHb and nitrate with a rate constant of 5–8 × 107 M−1s−1 [25–28]. MetHb binds nitrite reversibly with a dissociation constant of 1 mM [98] or lower under certain conditions [102]. In our systems, peroxide formed by reaction 2 appears to be scavenged by catalase (reaction 4 where the initial step in this reaction occurs with a rate constant greater than 107 M−1s−1 [95]). HNO also reacts with metHb (reaction 5) to form HbNO, with a rate constant that is fast (8 × 105 M−1s−1(based on that reported for metmyoglobin [85])), but significantly slower than reaction 2 with oxyHb. HbNO reacts slowly with oxygen to form metHb (reaction 6) with a rate that may be governed by the dissociation rate constant of NO (about 10−3 s−1 for some dissociations [107]). HNO also reacts with catalase (reaction 7 occurring with a rate constant of 3 × 105 M−1s−1 [85]) to form a ferrous NO complex. The NO-catalase can release NO relatively rapidly [97] which can then make metHb from oxyHb via the reaction described in Equation 1 (reaction 9) or react with oxygen (reaction 8 occurring at about 5 × 10−4 M−1s−1 [97]). Finally, HNO can react with low molecular weight thiols (reaction 10) with a rate constant of 2 × 106 M−1s−1 [85]. HNO can also react with protein bound thiols but this reaction is likely to be slower.
