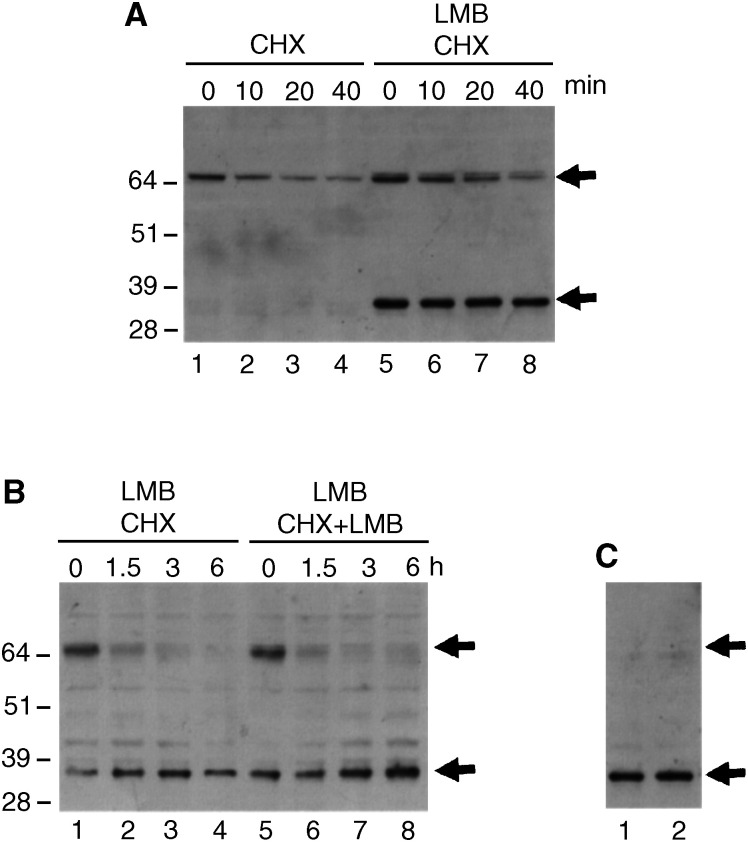
Figure 4.
Analysis of the stability of hMdm2 in the presence of LMB. (A) H1299 cells were transfected with the expression vector for hMdm2 and left untreated (lanes 1–4) or pretreated with 2 nM LMB for 18 h (lanes 5–8). After this time, 10 μg ml−1 cyclohexamide (CHX) was added to the medium and the cells were harvested after 0, 10, 20 and 40 min. Cell extracts were analysed by Western blotting and hMdm2 was detected using 4B2. The positions of the bands corresponding to the full-length hMdm2 and the 32 kDa fragment are indicated by arrows. (B) H1299 cells were transfected with the expression vector for hMdm2 and pretreated with 2 nM LMB for 18 h. After this time, the medium was removed and replaced with a medium containing 10 μg ml−1 CHX (lanes 1–4) or a medium containing 10 μg ml−1 CHX and 2 nM LMB (lanes 5–8). Cells were harvested after 0, 1.5, 3 and 6 h and cell extracts were analysed by Western blotting and hMdm2 was detected using 4B2. (C) H1299 cells were transfected with the expression vector for hMdm2 and pretreated with 2 nM LMB for 18 h after which 10 μg ml−1 CHX was added for 4 h. At this time, virtually all detected hMdm2 has an apparent molecular weight of 32 kDa. In order to see whether removal of LMB destabilised the 32 kDa fragment, we removed the medium and replaced it with a medium containing 10 μg ml−1 CHX only (lane 1) or a medium containing 10 μg ml−1 CHX and 2 nM LMB (lane 2). After 5 more h, cells were harvested and analysed as above.