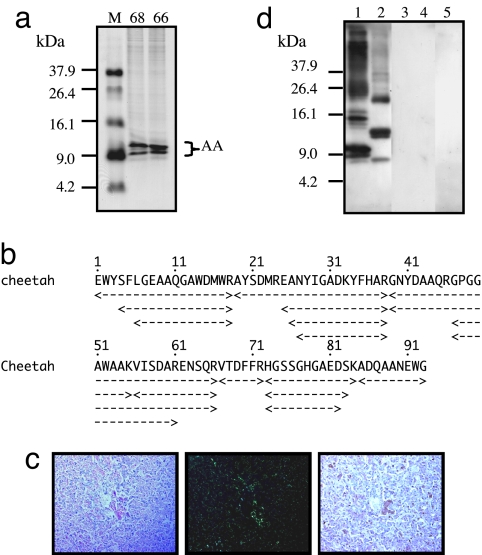
Fig. 1.
Cheetah AA amyloid fibril proteins and specific antiserum. (a) The isolated AA amyloid fibril fractions from the livers of C68 and C66 (20 μg) were applied to 16.5% Tris·tricine SDS/PAGE gel followed by CBB staining. M, molecular weight markers. Molecular masses of two main and two faint bands corresponded to the AA monomer. (b) Amino acid sequencing from four bands in C68 sample. AA amyloid fragments with different lengths were obtained. The amyloid fibril fraction from C68 was used as antigen to produce specific anti-cheetah AA antiserum. (c) Amyloid deposition in the liver of C68 was detected in Congo red-stained sections by standard microscopy (Left), by green birefringence under polarized microscopy (Center), and by immunohistochemical staining (Right). The anti-cheetah AA antiserum was used as primary antibody (dilution 1:1,000). (Magnification, ×200.) (d) AA amyloid fibril fractions from the liver of C68 (lane 1), human (lane 2), mouse (lane 3), cow (lane 4), and murine AApoAII(C) amyloid fibril fraction (lane 5) were used (12 μg of protein per well) to perform Western blot analysis with anti-cheetah AA antiserum as primary antibody (dilution 1:3,000).