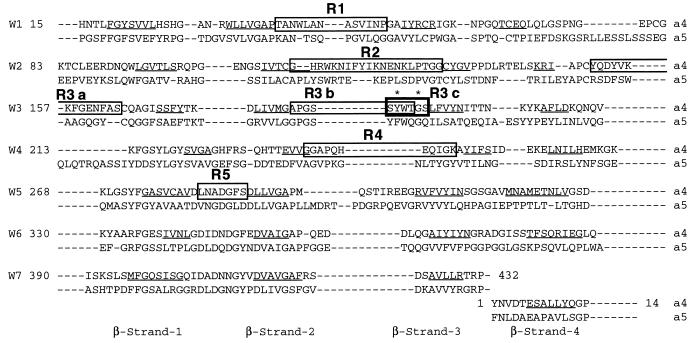
Figure 1.
Regions of α4 selected for swapping in this study. Integrin α subunits have seven repeats of about 60 amino acid residues each at their N terminals. We chose predicted loop structures within or close to the previously identified putative ligand-binding sites (residues 108–268) (11) for swapping with the corresponding residues of α5 (boxed regions). W1–W7 represent repeats 1–7 (14). The predicted β-strands of α4 are underlined (14). ∗, Tyr-187 and Gly-190 of α4, which are critical for ligand binding (12).