Abstract
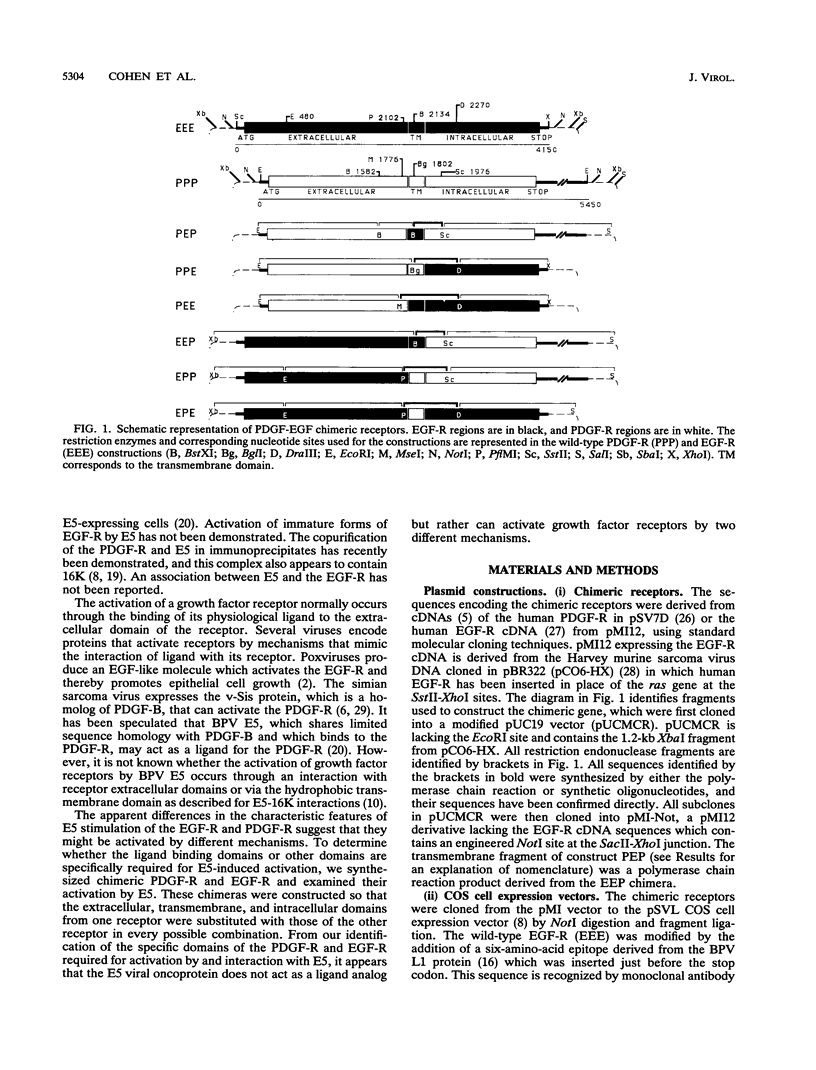
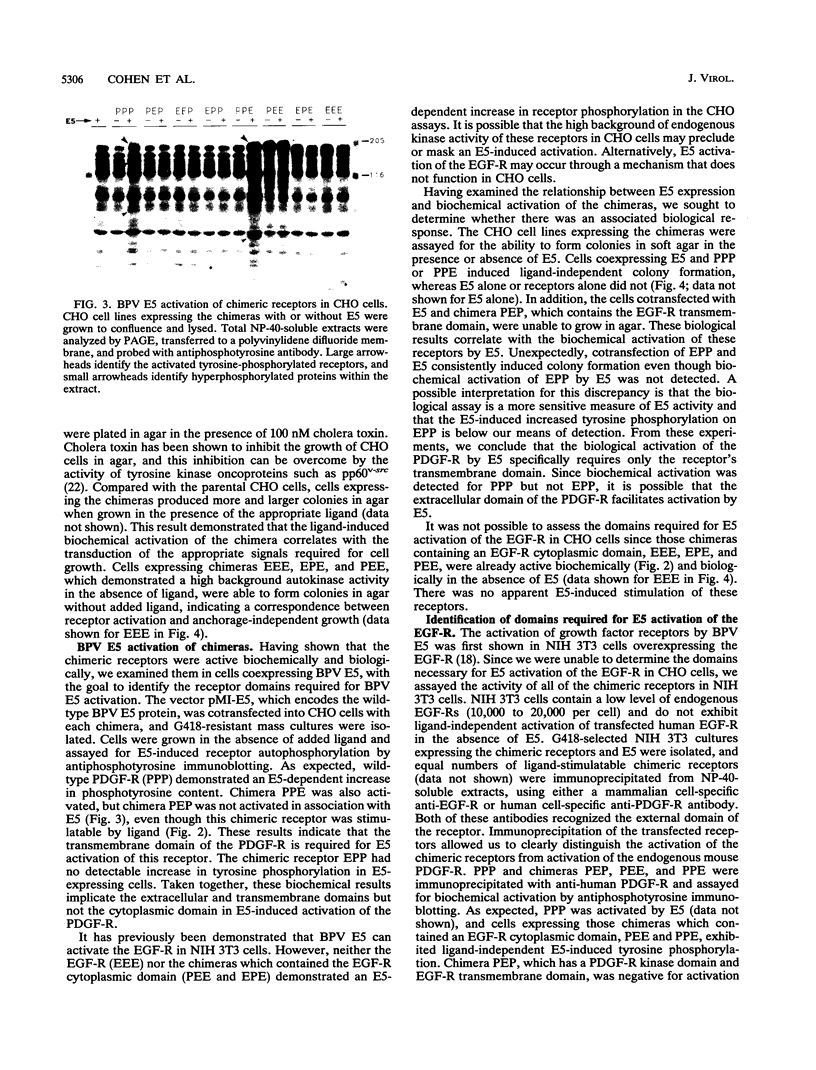
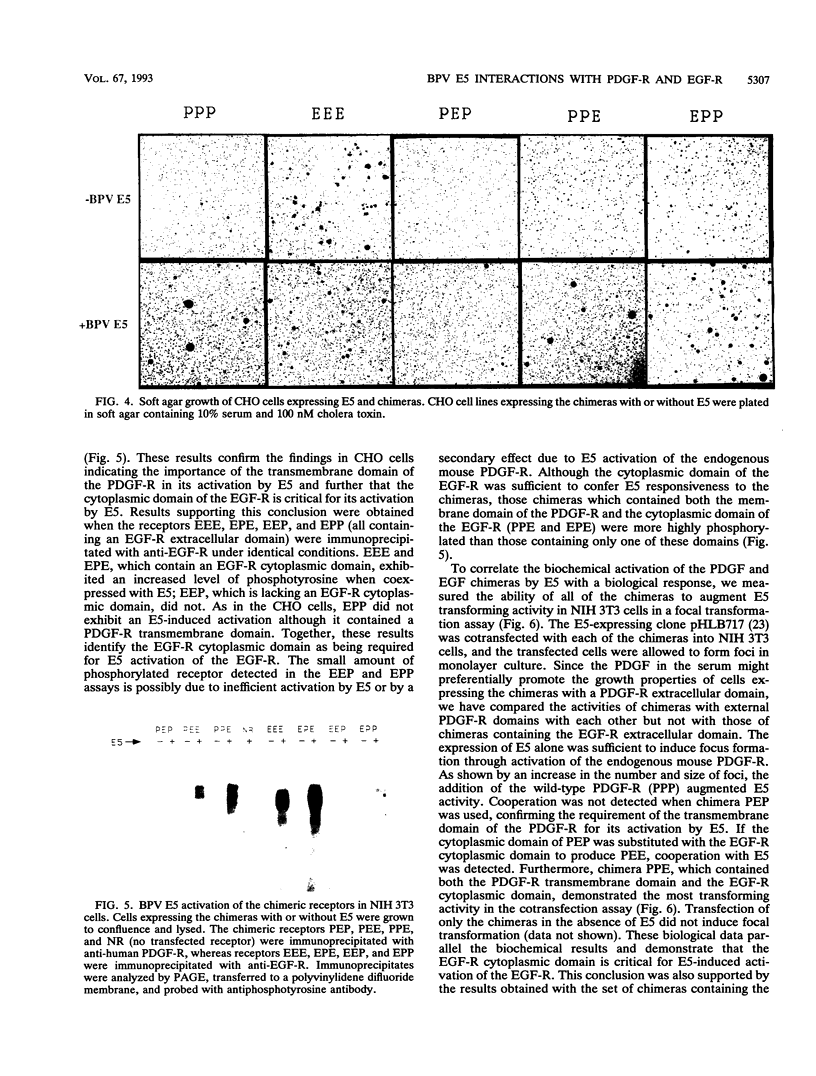
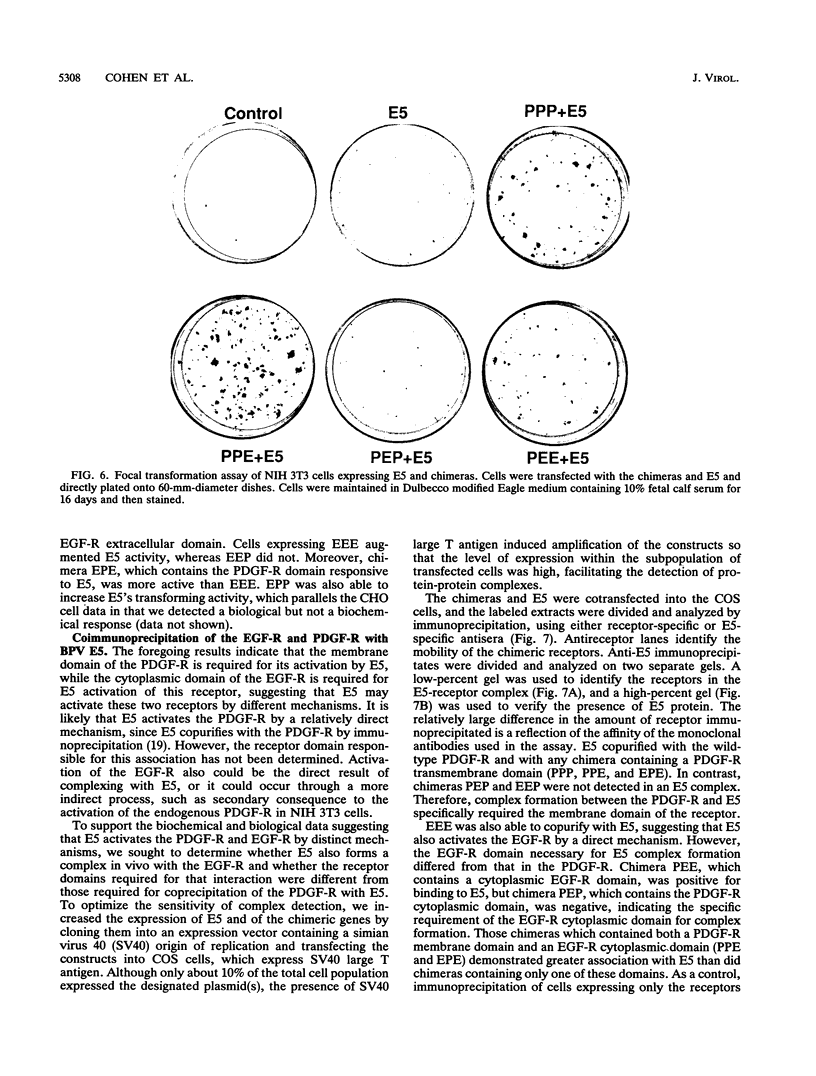
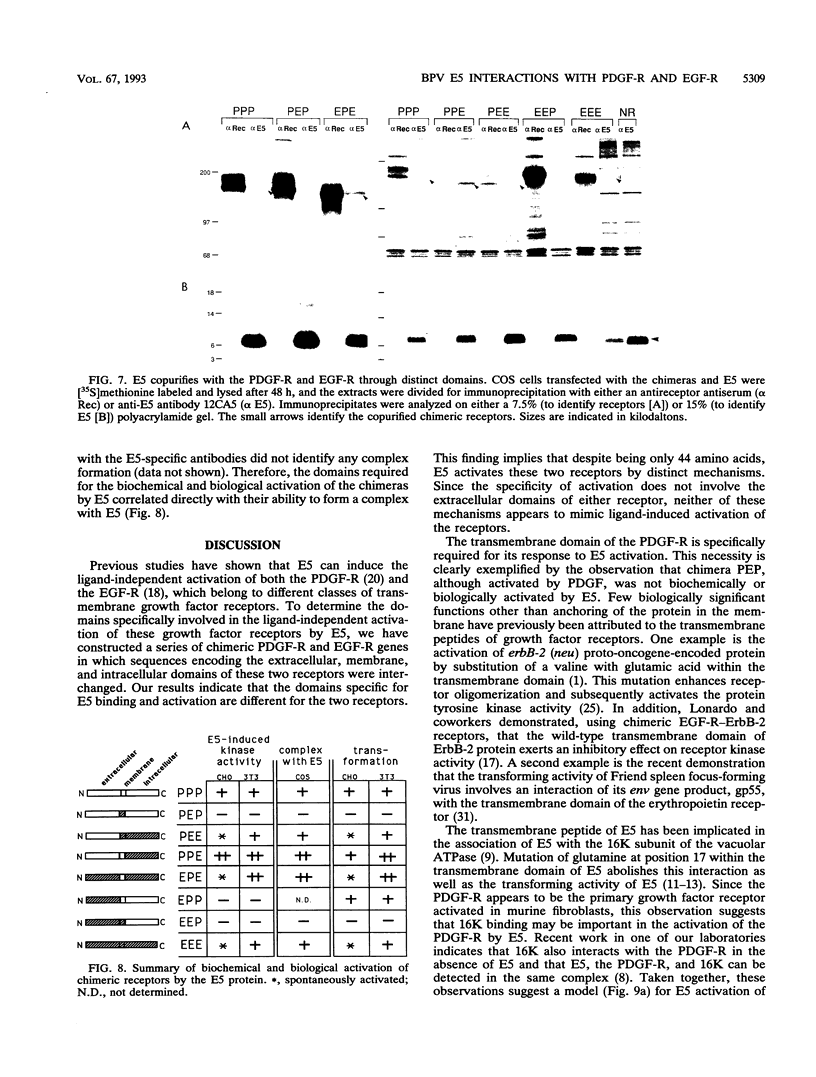
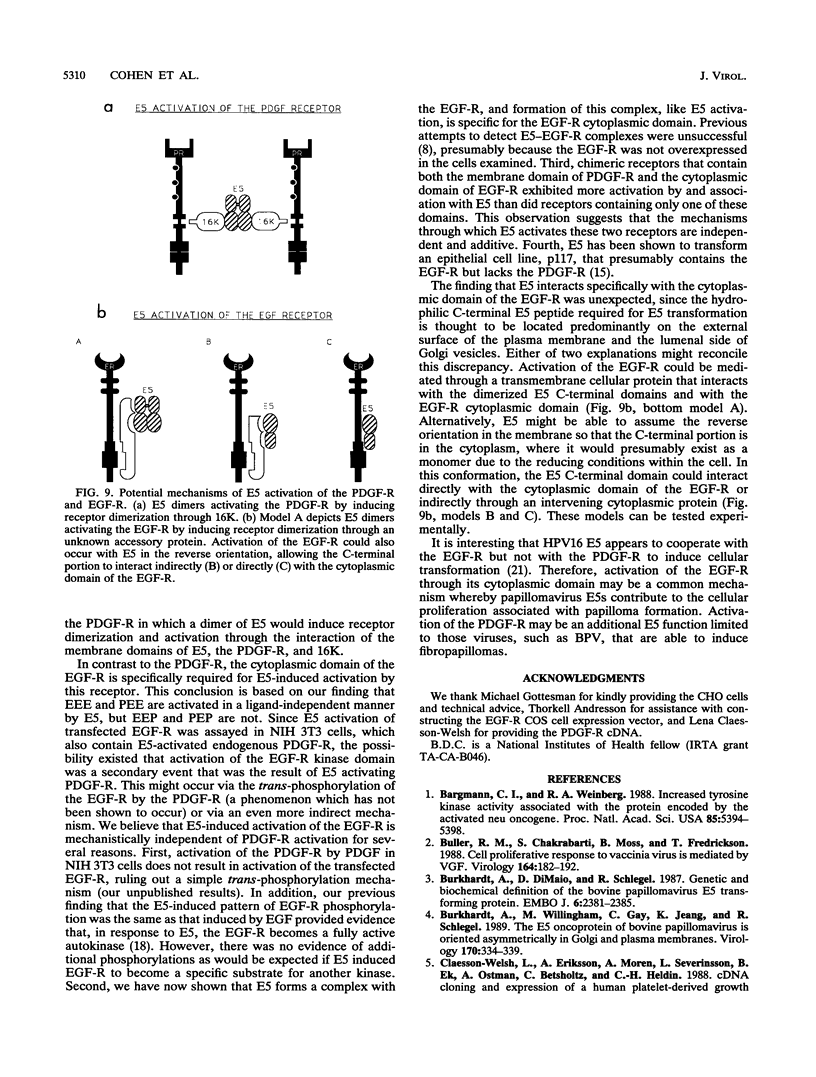
The bovine papillomavirus E5 transforming protein appears to activate both the epidermal growth factor receptor (EGF-R) and the platelet-derived growth factor receptor (PDGF-R) by a ligand-independent mechanism. To further investigate the ability of E5 to activate receptors of different classes and to determine whether this stimulation occurs through the extracellular domain required for ligand activation, we constructed chimeric genes encoding PDGF-R and EGF-R by interchanging the extracellular, membrane, and cytoplasmic coding domains. Chimeras were transfected into NIH 3T3 and CHO(LR73) cells. All chimeras expressed stable protein which, upon addition of the appropriate ligand, could be activated as assayed by tyrosine autophosphorylation and biological transformation. Cotransfection of E5 with the wild-type and chimeric receptors resulted in the ligand-independent activation of receptors, provided that a receptor contained either the transmembrane domain of the PDGF-R or the cytoplasmic domain of the EGF-R. Chimeric receptors that contained both of these domains exhibited the highest level of E5-induced biochemical and biological stimulation. These results imply that E5 activates the PDGF-R and EGR-R by two distinct mechanisms, neither of which specifically involves the extracellular domain of the receptor. Consistent with the biochemical and biological activation data, coimmunoprecipitation studies demonstrated that E5 formed a complex with any chimera that contained a PDGF-R transmembrane domain or an EGF-R cytoplasmic domain, with those chimeras containing both domains demonstrating the greatest efficiency of complex formation. These results suggest that although different domains of the PDGF-R and EGF-R are required for E5 activation, both receptors are activated directly by formation of an E5-containing complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller R. M., Chakrabarti S., Moss B., Fredrickson T. Cell proliferative response to vaccinia virus is mediated by VGF. Virology. 1988 May;164(1):182–192. doi: 10.1016/0042-6822(88)90635-6. [DOI] [PubMed] [Google Scholar]
- Burkhardt A., DiMaio D., Schlegel R. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 1987 Aug;6(8):2381–2385. doi: 10.1002/j.1460-2075.1987.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A., Willingham M., Gay C., Jeang K. T., Schlegel R. The E5 oncoprotein of bovine papillomavirus is oriented asymmetrically in Golgi and plasma membranes. Virology. 1989 May;170(1):334–339. doi: 10.1016/0042-6822(89)90391-7. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Dvoretzky I., Shober R., Chattopadhyay S. K., Lowy D. R. A quantitative in vitro focus assay for bovine papilloma virus. Virology. 1980 Jun;103(2):369–375. doi: 10.1016/0042-6822(80)90195-6. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Andresson T., Sparkowski J. J., Schlegel R. The BPV-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions. EMBO J. 1992 Dec;11(13):4851–4859. doi: 10.1002/j.1460-2075.1992.tb05591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Finbow M. E., Andresson T., McLean P., Smith K., Bubb V., Schlegel R. Bovine papillomavirus E5 oncoprotein binds to the 16K component of vacuolar H(+)-ATPases. Nature. 1991 Jul 25;352(6333):347–349. doi: 10.1038/352347a0. [DOI] [PubMed] [Google Scholar]
- Goldstein D. J., Kulke R., Dimaio D., Schlegel R. A glutamine residue in the membrane-associating domain of the bovine papillomavirus type 1 E5 oncoprotein mediates its binding to a transmembrane component of the vacuolar H(+)-ATPase. J Virol. 1992 Jan;66(1):405–413. doi: 10.1128/jvi.66.1.405-413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein D. J., Schlegel R. The E5 oncoprotein of bovine papillomavirus binds to a 16 kd cellular protein. EMBO J. 1990 Jan;9(1):137–145. doi: 10.1002/j.1460-2075.1990.tb08089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Burkhardt A. L., Schlegel R., DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol Cell Biol. 1988 Oct;8(10):4071–4078. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Weinstat D. L., DiMaio D. Transforming activity of a 16-amino-acid segment of the bovine papillomavirus E5 protein linked to random sequences of hydrophobic amino acids. J Virol. 1989 Nov;63(11):4515–4519. doi: 10.1128/jvi.63.11.4515-4519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C. Animal papillomaviruses. Microbiol Rev. 1982 Jun;46(2):191–207. doi: 10.1128/mr.46.2.191-207.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leptak C., Ramon y Cajal S., Kulke R., Horwitz B. H., Riese D. J., 2nd, Dotto G. P., DiMaio D. Tumorigenic transformation of murine keratinocytes by the E5 genes of bovine papillomavirus type 1 and human papillomavirus type 16. J Virol. 1991 Dec;65(12):7078–7083. doi: 10.1128/jvi.65.12.7078-7083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P. S., Jenson A. B., Cowsert L., Nakai Y., Lim L. Y., Jin X. W., Sundberg J. P. Distribution and specific identification of papillomavirus major capsid protein epitopes by immunocytochemistry and epitope scanning of synthetic peptides. J Infect Dis. 1990 Dec;162(6):1263–1269. doi: 10.1093/infdis/162.6.1263. [DOI] [PubMed] [Google Scholar]
- Lonardo F., Di Marco E., King C. R., Pierce J. H., Segatto O., Aaronson S. A., Di Fiore P. P. The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol. 1990 Nov;2(11):992–1003. [PubMed] [Google Scholar]
- Martin P., Vass W. C., Schiller J. T., Lowy D. R., Velu T. J. The bovine papillomavirus E5 transforming protein can stimulate the transforming activity of EGF and CSF-1 receptors. Cell. 1989 Oct 6;59(1):21–32. doi: 10.1016/0092-8674(89)90866-0. [DOI] [PubMed] [Google Scholar]
- Petti L., DiMaio D. Stable association between the bovine papillomavirus E5 transforming protein and activated platelet-derived growth factor receptor in transformed mouse cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6736–6740. doi: 10.1073/pnas.89.15.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petti L., Nilson L. A., DiMaio D. Activation of the platelet-derived growth factor receptor by the bovine papillomavirus E5 transforming protein. EMBO J. 1991 Apr;10(4):845–855. doi: 10.1002/j.1460-2075.1991.tb08017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pim D., Collins M., Banks L. Human papillomavirus type 16 E5 gene stimulates the transforming activity of the epidermal growth factor receptor. Oncogene. 1992 Jan;7(1):27–32. [PubMed] [Google Scholar]
- Roth C. W., Singh T., Pastan I., Gottesman M. M. Rous sarcoma virus transformed cells are resistant to cyclic AMP. J Cell Physiol. 1982 Apr;111(1):42–48. doi: 10.1002/jcp.1041110108. [DOI] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Wade-Glass M., Rabson M. S., Yang Y. C. The E5 transforming gene of bovine papillomavirus encodes a small, hydrophobic polypeptide. Science. 1986 Jul 25;233(4762):464–467. doi: 10.1126/science.3014660. [DOI] [PubMed] [Google Scholar]
- Sternberg M. J., Gullick W. J. Neu receptor dimerization. Nature. 1989 Jun 22;339(6226):587–587. doi: 10.1038/339587a0. [DOI] [PubMed] [Google Scholar]
- Truett M. A., Blacher R., Burke R. L., Caput D., Chu C., Dina D., Hartog K., Kuo C. H., Masiarz F. R., Merryweather J. P. Characterization of the polypeptide composition of human factor VIII:C and the nucleotide sequence and expression of the human kidney cDNA. DNA. 1985 Oct;4(5):333–349. doi: 10.1089/dna.1985.4.333. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Zhang K., Pastan I., Lowy D. R. Retroviruses expressing different levels of the normal epidermal growth factor receptor: biological properties and new bioassay. J Cell Biochem. 1989 Feb;39(2):153–166. doi: 10.1002/jcb.240390207. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Waters C. M., Overholser K. A., Sorkin A., Carpenter G. Analysis of the influences of the E5 transforming protein on kinetic parameters of epidermal growth factor binding and metabolism. J Cell Physiol. 1992 Aug;152(2):253–263. doi: 10.1002/jcp.1041520206. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Moreau J. F., Koo J. W., Mathey-Prevot B., D'Andrea A. D. The erythropoietin receptor transmembrane region is necessary for activation by the Friend spleen focus-forming virus gp55 glycoprotein. Mol Cell Biol. 1992 Jul;12(7):2949–2957. doi: 10.1128/mcb.12.7.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]