Abstract
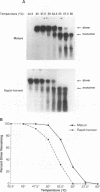
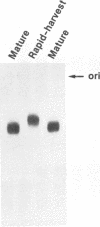
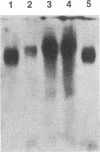
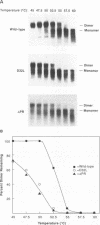
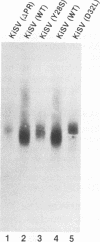
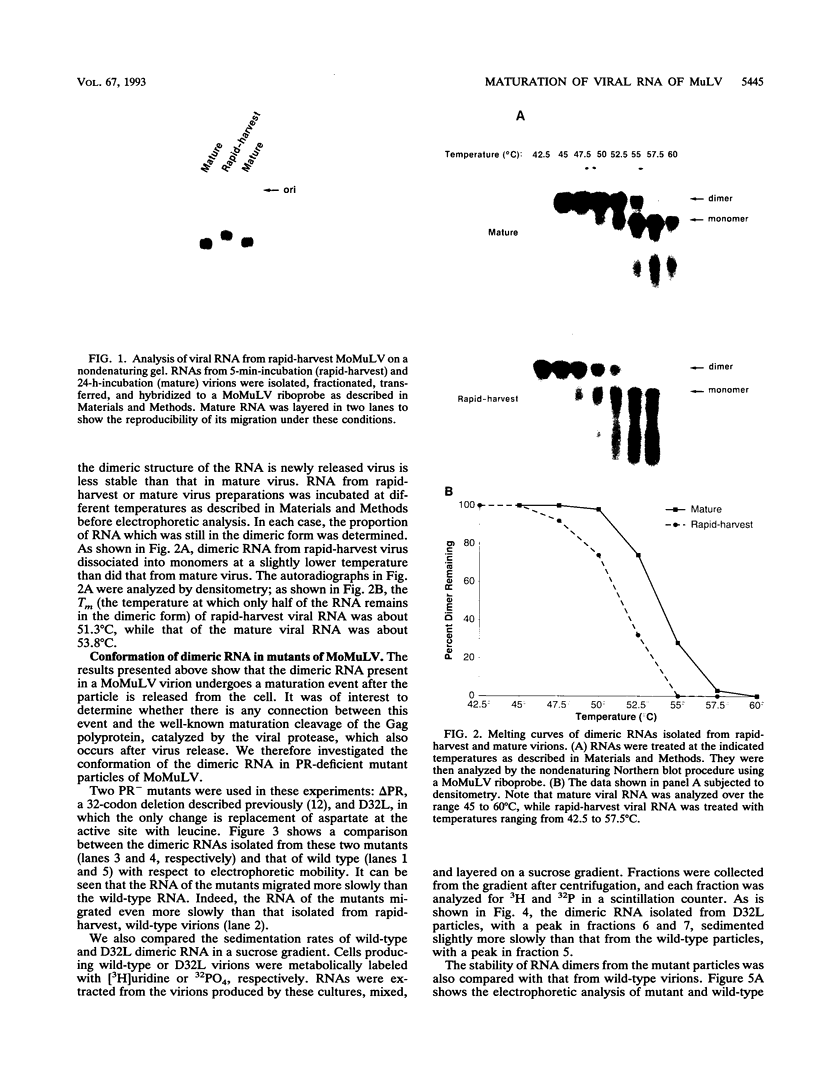
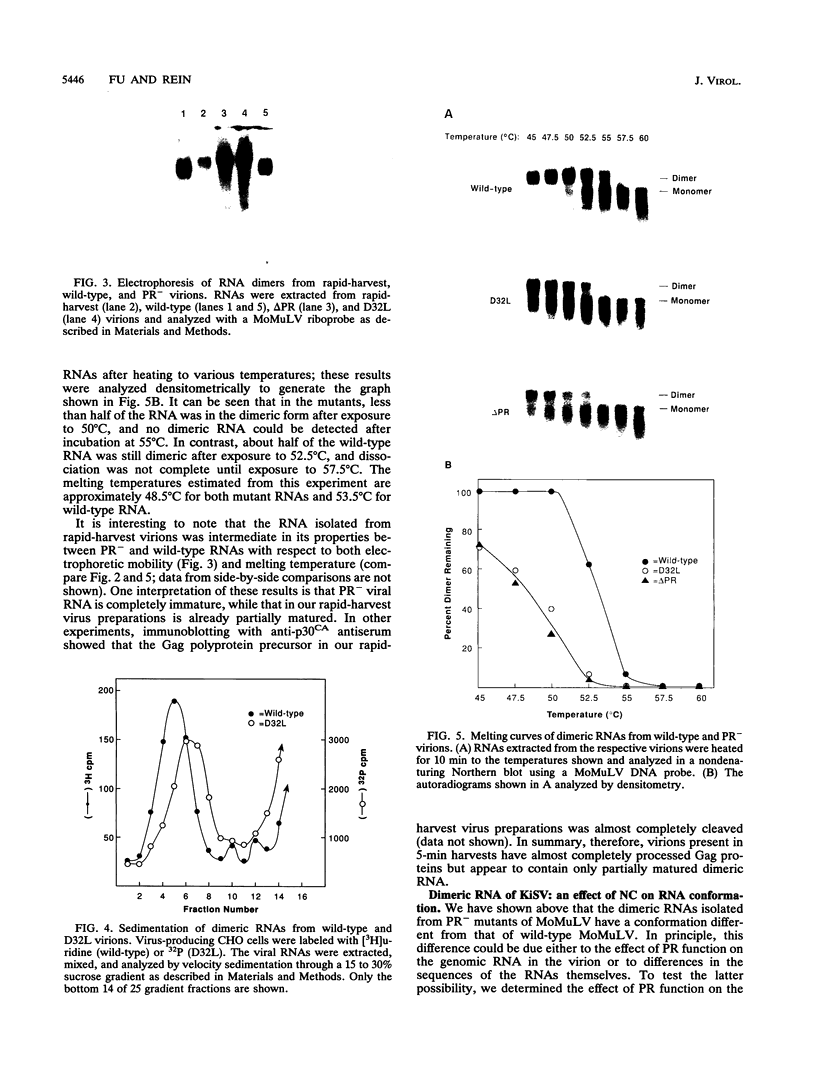
We have analyzed the dimeric RNA present in Moloney murine leukemia virus (MoMuLV) particles. We found that the RNA in newly released virions is in a conformation different from that in mature virions, since it has a different electrophoretic mobility in nondenaturing agarose gels and dissociates into monomers at a lower temperature. On the basis of these results, we suggest that the RNA initially packaged into nascent virions is already dimeric but that the dimer undergoes a maturation process after the virus is released from the cell. In further experiments, we tested the possibility that this maturation event is linked to the maturation cleavage of the virion proteins, which is catalyzed by the viral protease (PR). We found that the dimeric RNA isolated from PR- mutant virions resembles that from immature virions: it has a lower electrophoretic mobility and a lower sedimentation rate, and it also dissociates at a lower temperature than does RNA from mature wild-type virions. When Kirsten sarcoma virus is rescued by a PR- mutant or by a somewhat leaky cysteine array mutant of MoMuLV, its RNA also exhibits a electrophoretic mobility lower than that in the wild-type pseudotype. These results suggest that the maturation of dimeric RNA in released virus particles requires the cleavage of the Gag precursor and the presence of an intact cysteine array in the released nucleocapsid protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M. A., Miller A. D. Identification of a signal in a murine retrovirus that is sufficient for packaging of nonretroviral RNA into virions. J Virol. 1988 Oct;62(10):3802–3806. doi: 10.1128/jvi.62.10.3802-3806.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff R., Hajjar A. M., Linial M. L. Avian retroviral RNA encapsidation: reexamination of functional 5' RNA sequences and the role of nucleocapsid Cys-His motifs. J Virol. 1993 Jan;67(1):178–188. doi: 10.1128/jvi.67.1.178-188.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P., Steck T. L. Analysis of the ribonucleic acid of murine leukemia virus. J Virol. 1969 Oct;4(4):454–459. doi: 10.1128/jvi.4.4.454-459.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles N. E., Damay P., Spahr P. F. Effect of rearrangements and duplications of the Cys-His motifs of Rous sarcoma virus nucleocapsid protein. J Virol. 1993 Feb;67(2):623–631. doi: 10.1128/jvi.67.2.623-631.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Duesberg P. Role of subunits of 60 to 70S avian tumor virus ribonucleic acid in its template activity for the viral deoxyribonucleic acid polymerase. J Virol. 1972 Jul;10(1):23–31. doi: 10.1128/jvi.10.1.23-31.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaani E., Helm K. V., Duesberg P. Evidence for 30-40S RNA as precursor of the 60-70S RNA of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1973 Feb;70(2):401–405. doi: 10.1073/pnas.70.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung K. S., Smith R. E., Stone M. P., Joklik W. K. Comparison of immature (rapid harvest) and mature Rous sarcoma virus particles. Virology. 1972 Dec;50(3):851–864. doi: 10.1016/0042-6822(72)90439-4. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Physical properties of Rous Sarcoma Virus RNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1511–1518. doi: 10.1073/pnas.60.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupraz P., Oertle S., Meric C., Damay P., Spahr P. F. Point mutations in the proximal Cys-His box of Rous sarcoma virus nucleocapsid protein. J Virol. 1990 Oct;64(10):4978–4987. doi: 10.1128/jvi.64.10.4978-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y. X., Hatfield D. L., Rein A., Levin J. G. Translational readthrough of the murine leukemia virus gag gene amber codon does not require virus-induced alteration of tRNA. J Virol. 1989 May;63(5):2405–2410. doi: 10.1128/jvi.63.5.2405-2410.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick R. J., Henderson L. E., Hanser J. P., Rein A. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a "zinc finger-like" protein sequence. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8420–8424. doi: 10.1073/pnas.85.22.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yoshinaka Y., Rein A., Shibuya M., Odaka T., Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from "immature" to "mature" core form and for virus infectivity. Virology. 1985 Sep;145(2):280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Méric C. A procedure for Northern blot analysis of native RNA. Anal Biochem. 1986 Nov 15;159(1):227–232. doi: 10.1016/0003-2697(86)90332-5. [DOI] [PubMed] [Google Scholar]
- Korb J., Trávnícek M., Ríman J. The oncornavirus maturation process: quantitative correlation between morphological changes and conversion of genomic virion RNA. Intervirology. 1976;7(4-5):211–224. doi: 10.1159/000149954. [DOI] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M. L., Miller A. D. Retroviral RNA packaging: sequence requirements and implications. Curr Top Microbiol Immunol. 1990;157:125–152. doi: 10.1007/978-3-642-75218-6_5. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McCoy M. S., Bargmann C. I., Weinberg R. A. Human colon carcinoma Ki-ras2 oncogene and its corresponding proto-oncogene. Mol Cell Biol. 1984 Aug;4(8):1577–1582. doi: 10.1128/mcb.4.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer L. I., Levin J. G., Chattopadhyay S. K. Metabolism of viral RNA in murine leukemia virus-infected cells; evidence for differential stability of viral message and virion precursor RNA. J Virol. 1981 Dec;40(3):683–690. doi: 10.1128/jvi.40.3.683-690.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Goff S. P. Characterization of Moloney murine leukemia virus mutants with single-amino-acid substitutions in the Cys-His box of the nucleocapsid protein. J Virol. 1989 Apr;63(4):1558–1568. doi: 10.1128/jvi.63.4.1558-1568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méric C., Spahr P. F. Rous sarcoma virus nucleic acid-binding protein p12 is necessary for viral 70S RNA dimer formation and packaging. J Virol. 1986 Nov;60(2):450–459. doi: 10.1128/jvi.60.2.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertle S., Spahr P. F. Role of the gag polyprotein precursor in packaging and maturation of Rous sarcoma virus genomic RNA. J Virol. 1990 Dec;64(12):5757–5763. doi: 10.1128/jvi.64.12.5757-5763.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats A. C., Roy C., Wang P. A., Erard M., Housset V., Gabus C., Paoletti C., Darlix J. L. cis elements and trans-acting factors involved in dimer formation of murine leukemia virus RNA. J Virol. 1990 Feb;64(2):774–783. doi: 10.1128/jvi.64.2.774-783.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart L., Schatz G., Vogt V. M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990 Oct;64(10):5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Snyder P. N. Structure of B77 sarcoma virus RNA: stabilization of RNA after packaging. J Virol. 1975 Nov;16(5):1161–1170. doi: 10.1128/jvi.16.5.1161-1170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]