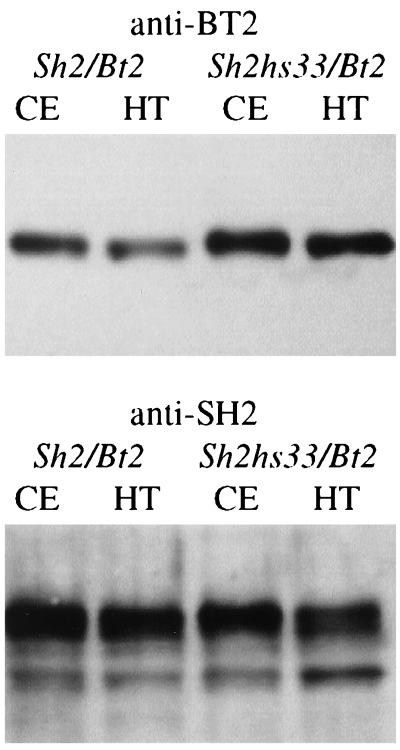
Figure 3.
Equivalent amounts of protein from nonheated (CE) and heated crude extracts (HT) were resolved through a 10% SDS/PAGE, blotted to nitrocellulose, and probed with antibody to either the BT2 or SH2 subunit as indicated above the blot. Results show that there is a significant increase in the level of BT2 antigen in the Sh2hs33/Bt2 mutant extract compared with the WT (Sh2/Bt2) extract. In contrast, comparable levels of SH2 antigen were detected in the Sh2/Bt2 and Sh2hs33/Bt2 nonheated and heated extracts. Results indicate the mutation in Sh2hs33 stabilizes or recruits the BT2 subunit more efficiently than does the WT SH2 protein.