Abstract
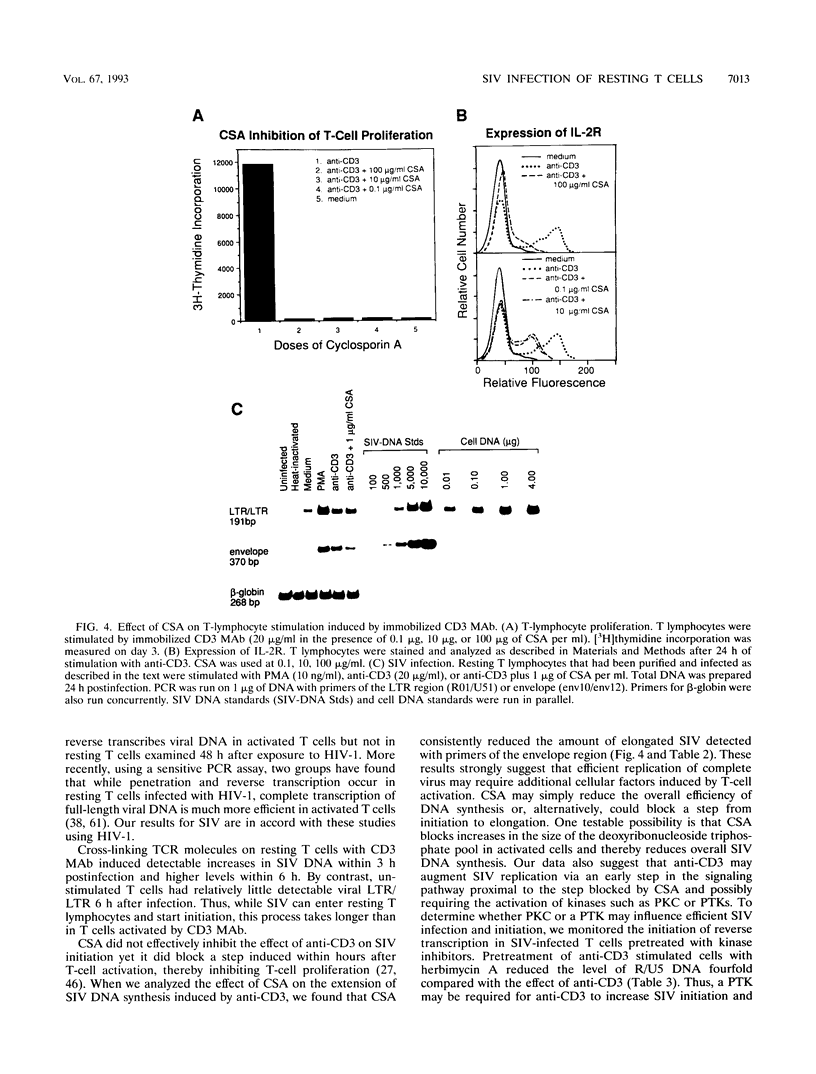
The relationship between T-cell activation and early events in the replication cycle of simian immunodeficiency virus (SIV) was analyzed in resting T lymphocytes from macaques. We used the polymerase chain reaction to detect an early product of reverse transcription (R/U5) and almost complete viral DNA (long terminal repeat/gag). We found that SIV can enter resting T lymphocytes and initiate replication but that the reverse transcription process is not efficient and proceeds slowly in resting cells. Cross-linking the CD3/T-cell receptor complex with monoclonal antibodies, unlike cross-linking either the CD28 or CD2 accessory receptor and like phorbol myristate acetate, induced a rapid increase in viral R/U5 DNA detected within 3 to 6 h postinfection. Anti-CD3 or phorbol myristate acetate induced replication of full-length viral DNA within 6 to 9 h postinfection, but full-length SIV DNA was not detectable at earlier time points. We then compared various inhibitors of T-cell activation for their effects on viral initiation and complete replication. Cyclosporin A, an inhibitor of a distal step in T-cell activation, blocked anti-CD3-induced T-cell proliferation and completion of SIV DNA replication but had no effect on induced increases in SIV R/U5 DNA. By contrast, initial SIV DNA synthesis was partially blocked by inhibitors of very early steps in T-cell activation, including herbimycin A, an inhibitor of protein tyrosine kinases, and by two different inhibitors of protein kinase C, H7 and staurosporine. Since resting T cells do not efficiently complete SIV DNA synthesis and cyclosporin A can block the formation of complete viral DNA induced in activated T cells, a cellular factor(s) present in activated T cells appears to be required for the formation of full-length SIV DNA.
Full text
PDF

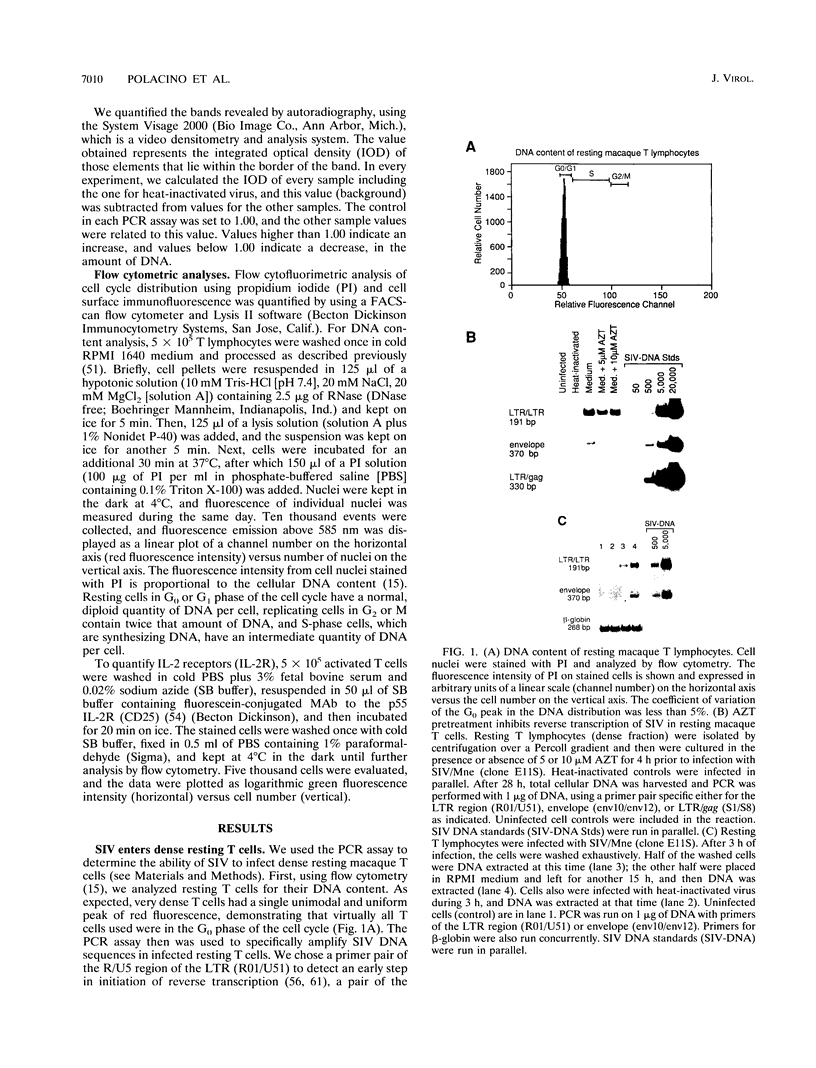





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Hill R. W., Eron L. J., Csaikl U. M., Knott W. B., Henderson L. E., Sowder R. C., Nagashima K., Gonda M. A. Characterization of clones of HIV-1 infected HuT 78 cells defective in gag gene processing and of SIV clones producing large amounts of envelope glycoprotein. J Med Primatol. 1990;19(3-4):351–366. [PubMed] [Google Scholar]
- Benveniste R. E., Morton W. R., Clark E. A., Tsai C. C., Ochs H. D., Ward J. M., Kuller L., Knott W. B., Hill R. W., Gale M. J. Inoculation of baboons and macaques with simian immunodeficiency virus/Mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988 Jun;62(6):2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler P., Pantaleo G., Demaria A., Fauci A. S. Anti-CD2 receptor antibodies activate the HIV long terminal repeat in T lymphocytes. J Immunol. 1991 Oct 1;147(7):2290–2294. [PubMed] [Google Scholar]
- Bukrinsky M. I., Stanwick T. L., Dempsey M. P., Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991 Oct 18;254(5030):423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann A. J., Zack J. A., Go A. S., Arrigo S. J., Koyanagi Y., Green P. L., Koyanagi Y., Pang S., Chen I. S. Human immunodeficiency virus type 1 T-cell tropism is determined by events prior to provirus formation. J Virol. 1990 Oct;64(10):4735–4742. doi: 10.1128/jvi.64.10.4735-4742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti L., Guyader M., Alizon M., Daniel M. D., Desrosiers R. C., Tiollais P., Sonigo P. Sequence of simian immunodeficiency virus from macaque and its relationship to other human and simian retroviruses. Nature. 1987 Aug 6;328(6130):543–547. doi: 10.1038/328543a0. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Weiss A. New insights into T-cell antigen receptor structure and signal transduction. Curr Opin Immunol. 1992 Jun;4(3):246–251. doi: 10.1016/0952-7915(92)90072-m. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Shu G., Ledbetter J. A. Role of the Bp35 cell surface polypeptide in human B-cell activation. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1766–1770. doi: 10.1073/pnas.82.6.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H., Alarcon B., Wileman T., Terhorst C. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol. 1988;6:629–662. doi: 10.1146/annurev.iy.06.040188.003213. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Letvin N. L., King N. W., Kannagi M., Sehgal P. K., Hunt R. D., Kanki P. J., Essex M., Desrosiers R. C. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985 Jun 7;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Fields A. P., Bednarik D. P., Hess A., May W. S. Human immunodeficiency virus induces phosphorylation of its cell surface receptor. Nature. 1988 May 19;333(6170):278–280. doi: 10.1038/333278a0. [DOI] [PubMed] [Google Scholar]
- Firpo P. P., Axberg I., Scheibel M., Clark E. A. Macaque CD4+ T-cell subsets: influence of activation on infection by simian immunodeficiency viruses (SIV). AIDS Res Hum Retroviruses. 1992 Mar;8(3):357–366. doi: 10.1089/aid.1992.8.357. [DOI] [PubMed] [Google Scholar]
- Franchini G., Gurgo C., Guo H. G., Gallo R. C., Collalti E., Fargnoli K. A., Hall L. F., Wong-Staal F., Reitz M. S., Jr Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature. 1987 Aug 6;328(6130):539–543. doi: 10.1038/328539a0. [DOI] [PubMed] [Google Scholar]
- Fried J., Perez A. G., Clarkson B. D. Flow cytofluorometric analysis of cell cycle distributions using propidium iodide. Properties of the method and mathematical analysis of the data. J Cell Biol. 1976 Oct;71(1):172–181. doi: 10.1083/jcb.71.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M. J., Jr, Ledbetter J. A., Schieven G. L., Jonker M., Morton W. R., Benveniste R. E., Clark E. A. CD4 and CD8 T cells from SIV-infected macaques have defective signaling responses after perturbation of either CD3 or CD2 receptors. Int Immunol. 1990;2(9):849–858. doi: 10.1093/intimm/2.9.849. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Gale M. J., Jr, Hoffman P. A., Willerford D. M., Draves K. E., Benveniste R. E., Morton W. R., Clark E. A. Selective replication of simian immunodeficiency virus in a subset of CD4+ lymphocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3301–3305. doi: 10.1073/pnas.86.9.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton G. N., Brass L. F., Kozbor D., Pletcher C. H., Hoxie J. A. Inhibition of T cell antigen receptor-dependent phosphorylation of CD4 in human immunodeficiency virus type 1 infected cells. J Biol Chem. 1992 Feb 25;267(6):4102–4109. [PubMed] [Google Scholar]
- Gowda S. D., Stein B. S., Mohagheghpour N., Benike C. J., Engleman E. G. Evidence that T cell activation is required for HIV-1 entry in CD4+ lymphocytes. J Immunol. 1989 Feb 1;142(3):773–780. [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruters R. A., Otto S. A., Al B. J., Verhoeven A. J., Verweij C. L., Van Lier R. A., Miedema F. Non-mitogenic T cell activation signals are sufficient for induction of human immunodeficiency virus transcription. Eur J Immunol. 1991 Jan;21(1):167–172. doi: 10.1002/eji.1830210125. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Abrams K., Misher L., Stallard V., Moran P., Zarling J. M., Langlois A. J., Kuller L., Morton W. R., Benveniste R. E. Evaluation of protective efficacy of recombinant subunit vaccines against simian immunodeficiency virus infection of macaques. J Med Primatol. 1992 Feb-May;21(2-3):119–125. [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Linsley P. S., Thompson C. B. Role of the CD28 receptor in T-cell activation. Immunol Today. 1990 Jun;11(6):211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Benzie C. R. Rapid loss of sensitivity of mitogen-induced lymphocyte activation to inhibition by cyclosporin A. Cell Immunol. 1984 Aug;87(1):217–224. doi: 10.1016/0008-8749(84)90145-x. [DOI] [PubMed] [Google Scholar]
- Kinter A. L., Poli G., Maury W., Folks T. M., Fauci A. S. Direct and cytokine-mediated activation of protein kinase C induces human immunodeficiency virus expression in chronically infected promonocytic cells. J Virol. 1990 Sep;64(9):4306–4312. doi: 10.1128/jvi.64.9.4306-4312.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld H., Cruikshank W. W., Pyle S. W., Berman J. S., Center D. M. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988 Sep 29;335(6189):445–448. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Lori F., di Marzo Veronese F., de Vico A. L., Lusso P., Reitz M. S., Jr, Gallo R. C. Viral DNA carried by human immunodeficiency virus type 1 virions. J Virol. 1992 Aug;66(8):5067–5074. doi: 10.1128/jvi.66.8.5067-5074.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Maggi E., Macchia D., Parronchi P., Mazzetti M., Ravina A., Milo D., Romagnani S. Reduced production of interleukin 2 and interferon-gamma and enhanced helper activity for IgG synthesis by cloned CD4+ T cells from patients with AIDS. Eur J Immunol. 1987 Dec;17(12):1685–1690. doi: 10.1002/eji.1830171202. [DOI] [PubMed] [Google Scholar]
- Mohagheghpour N., Chakrabarti R., Stein B. S., Gowda S. D., Engleman E. G. Early activation events render T cells susceptible to HIV-1-induced syncytia formation. Role of protein kinase C. J Biol Chem. 1991 Apr 15;266(11):7233–7238. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Nooij F. J., Borst J. G., Van Meurs G. J., Jonker M., Balner H. Differentiation antigens on rhesus monkey lymphocytes. I. Identification of T cells bearing CD3 and CD8, and of a subset of CD8-bearing cells. Eur J Immunol. 1986 Aug;16(8):975–979. doi: 10.1002/eji.1830160817. [DOI] [PubMed] [Google Scholar]
- Orloff G. M., Orloff S. L., Kennedy M. S., Maddon P. J., McDougal J. S. Penetration of CD4 T cells by HIV-1. The CD4 receptor does not internalize with HIV, and CD4-related signal transduction events are not required for entry. J Immunol. 1991 Apr 15;146(8):2578–2587. [PubMed] [Google Scholar]
- Overbaugh J., Rudensey L. M., Papenhausen M. D., Benveniste R. E., Morton W. R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991 Dec;65(12):7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli G., Kinter A., Justement J. S., Kehrl J. H., Bressler P., Stanley S., Fauci A. S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Induction of expression of HIV in latently or chronically infected cells. AIDS Res Hum Retroviruses. 1989 Feb;5(1):1–4. doi: 10.1089/aid.1989.5.1. [DOI] [PubMed] [Google Scholar]
- Rudensey L. M., Papenhausen M. D., Overbaugh J. Replication and persistence of simian immunodeficiency virus variants after passage in macaque lymphocytes and established human cell lines. J Virol. 1993 Mar;67(3):1727–1733. doi: 10.1128/jvi.67.3.1727-1733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savatier P., Trabuchet G., Chebloune Y., Faure C., Verdier G., Nigon V. M. Nucleotide sequence of the delta-beta-globin intergenic segment in the macaque: structure and evolutionary rates in higher primates. J Mol Evol. 1987;24(4):297–308. doi: 10.1007/BF02134128. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Lane H. C., Greenhouse J., Justement J. S., Baseler M., Fauci A. S. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N. H., Dumont F. J. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Sommermeyer H., Schwinzer R., Kaever V., Behl B., Resch K. The G protein coupling T cell antigen receptor/CD3-complex and phospholipase C in the human T cell lymphoma Jurkat is not a target for cholera toxin. Eur J Immunol. 1990 Sep;20(9):1881–1886. doi: 10.1002/eji.1830200902. [DOI] [PubMed] [Google Scholar]
- Srivastava K. K., Fernandez-Larsson R., Zinkus D. M., Robinson H. L. Human immunodeficiency virus type 1 NL4-3 replication in four T-cell lines: rate and efficiency of entry, a major determinant of permissiveness. J Virol. 1991 Jul;65(7):3900–3902. doi: 10.1128/jvi.65.7.3900-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M., Stanwick T. L., Dempsey M. P., Lamonica C. A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990 May;9(5):1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate E. H., Wilder M. E., Cram L. S., Wharton W. A method for staining 3T3 cell nuclei with propidium iodide in hypotonic solution. Cytometry. 1983 Nov;4(3):211–215. doi: 10.1002/cyto.990040304. [DOI] [PubMed] [Google Scholar]
- Tong-Starkesen S. E., Luciw P. A., Peterlin B. M. Signaling through T lymphocyte surface proteins, TCR/CD3 and CD28, activates the HIV-1 long terminal repeat. J Immunol. 1989 Jan 15;142(2):702–707. [PubMed] [Google Scholar]
- Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992 Aug;66(8):4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Vicentini L. M., Villereal M. L. Activation of Na+/H+ exchange in cultured fibroblasts: synergism and antagonism between phorbol ester, Ca2+ ionophore, and growth factors. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8053–8056. doi: 10.1073/pnas.82.23.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta E. S., Berton M. T., Burger C., Kepron M., Lee W. T., Yin X. M. Memory B and T cells. Annu Rev Immunol. 1991;9:193–217. doi: 10.1146/annurev.iy.09.040191.001205. [DOI] [PubMed] [Google Scholar]
- Vyakarnam A., McKeating J., Meager A., Beverley P. C. Tumour necrosis factors (alpha, beta) induced by HIV-1 in peripheral blood mononuclear cells potentiate virus replication. AIDS. 1990 Jan;4(1):21–27. doi: 10.1097/00002030-199001000-00003. [DOI] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Hardy K., Manger B., Terhorst C., Stobo J. The role of the T3/antigen receptor complex in T-cell activation. Annu Rev Immunol. 1986;4:593–619. doi: 10.1146/annurev.iy.04.040186.003113. [DOI] [PubMed] [Google Scholar]
- Willerford D. M., Gale M. J., Jr, Benveniste R. E., Clark E. A., Gallatin W. M. Simian immunodeficiency virus is restricted to a subset of blood CD4+ lymphocytes that includes memory cells. J Immunol. 1990 May 15;144(10):3779–3783. [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Haislip A. M., Krogstad P., Chen I. S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992 Mar;66(3):1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier R. A., Brouwer M., Rebel V. I., van Noesel C. J., Aarden L. A. Immobilized anti-CD3 monoclonal antibodies induce accessory cell-independent lymphokine production, proliferation and helper activity in human T lymphocytes. Immunology. 1989 Sep;68(1):45–50. [PMC free article] [PubMed] [Google Scholar]