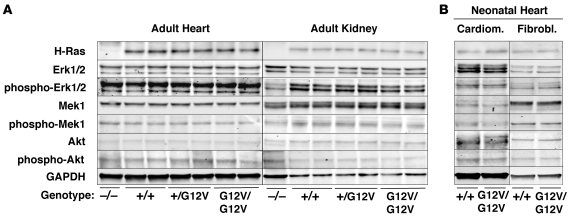
Figure 2. H-Ras signaling in heart and kidneys of H-RasG12V mutant mice.
Protein extracts (40 μg) obtained from (A) heart and kidneys of 2-month-old H-Ras–/–, H-Ras+/+, H-Ras+/G12V, and H-RasG12V/G12V mice and from (B) cardiomyocytes and fibroblasts isolated form neonatal hearts of H-Ras+/+ and H-RasG12V/G12V animals were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and blotted with antibodies against H-Ras and the nonphosphorylated and phosphorylated forms of Erk1/2, Mek1, and Akt. GAPDH was used as loading control.