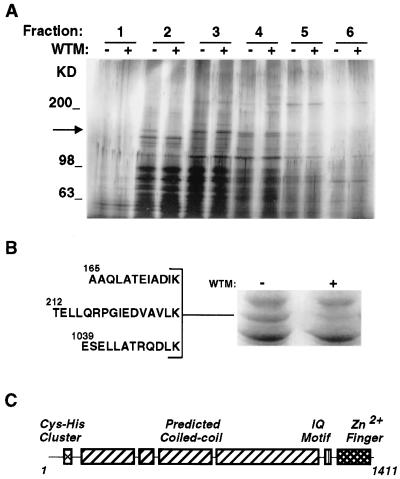
Figure 1.
(A) Peripheral membrane proteins from 3T3-L1 adipocyte membranes. 3T3-L1 adipocytes were treated without (−) or with (+) wortmannin (50 nM) for 15 min. Crude membrane fractions were prepared and peripheral membrane proteins released with 0.5 M KCl. Proteins were separated by velocity centrifugation on 10–30% sucrose gradients, and 10 fractions were collected. Fractions were analyzed by 5–15% SDS/PAGE and silver staining. The first six fractions are shown. The arrow indicates the position of a 170-kDa band, present in the second fraction, that is greatly reduced in extracts from wortmannin-treated cells. Molecular size markers are indicated to the right in kilodaltons. (B) Sequence of peptides derived from p170. Approximately 5 pmol of p170, obtained from untreated cells (right lane), were excised and subjected to lysyl endoproteinase digestion, separation, and sequencing by microcapillary HPLC-electrospray ionization tandem MS. The sequences of three peptides that aligned with the sequence of EEA1 are shown. (C) Domain structure of EEA1 (18).