Abstract
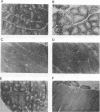
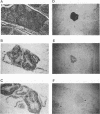
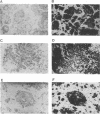
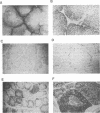
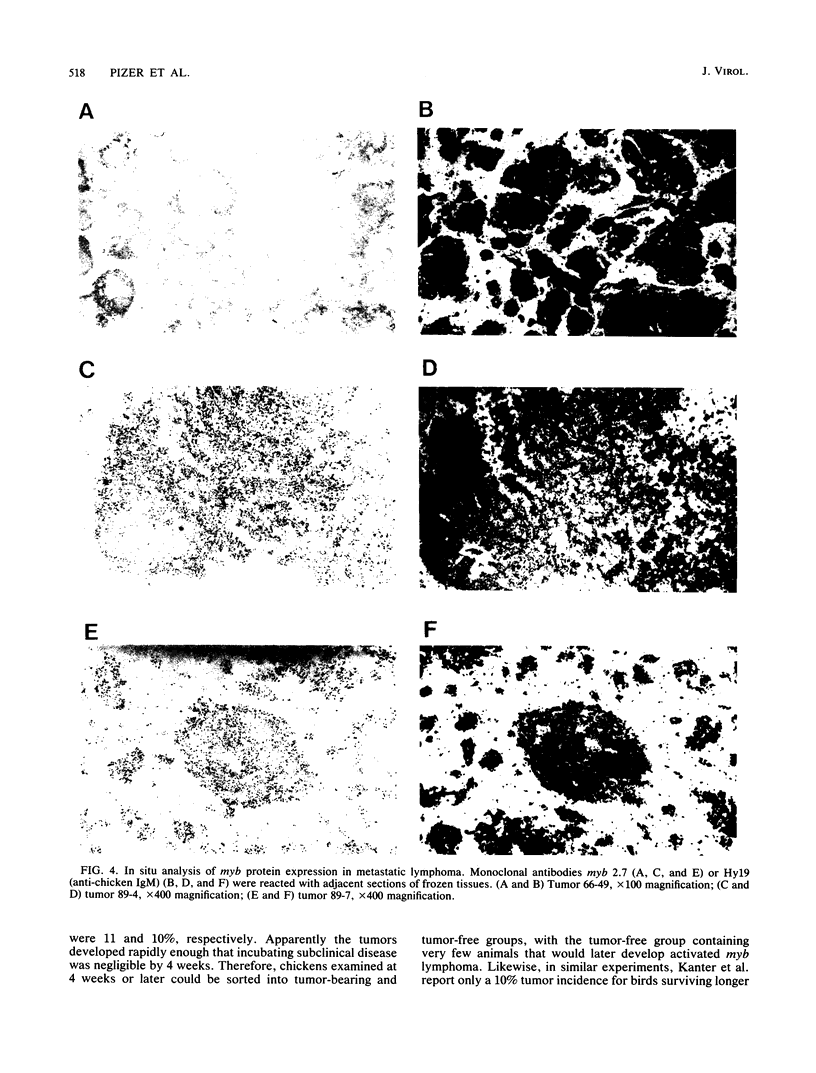
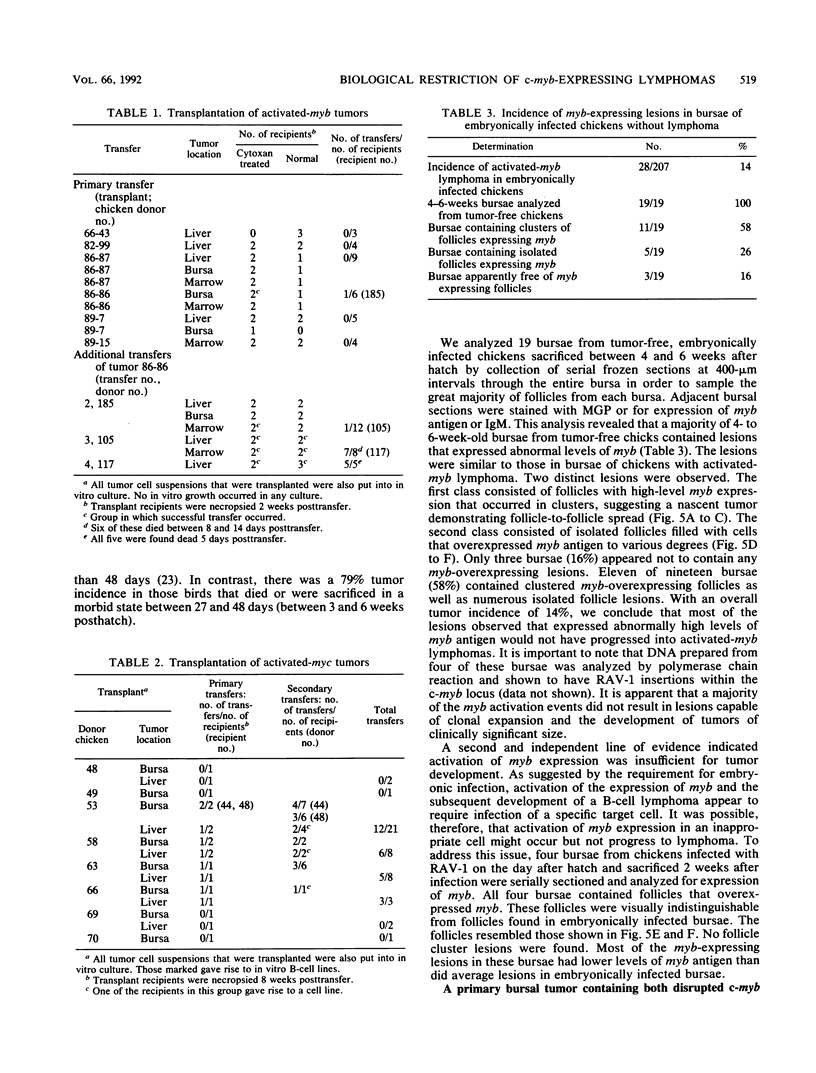
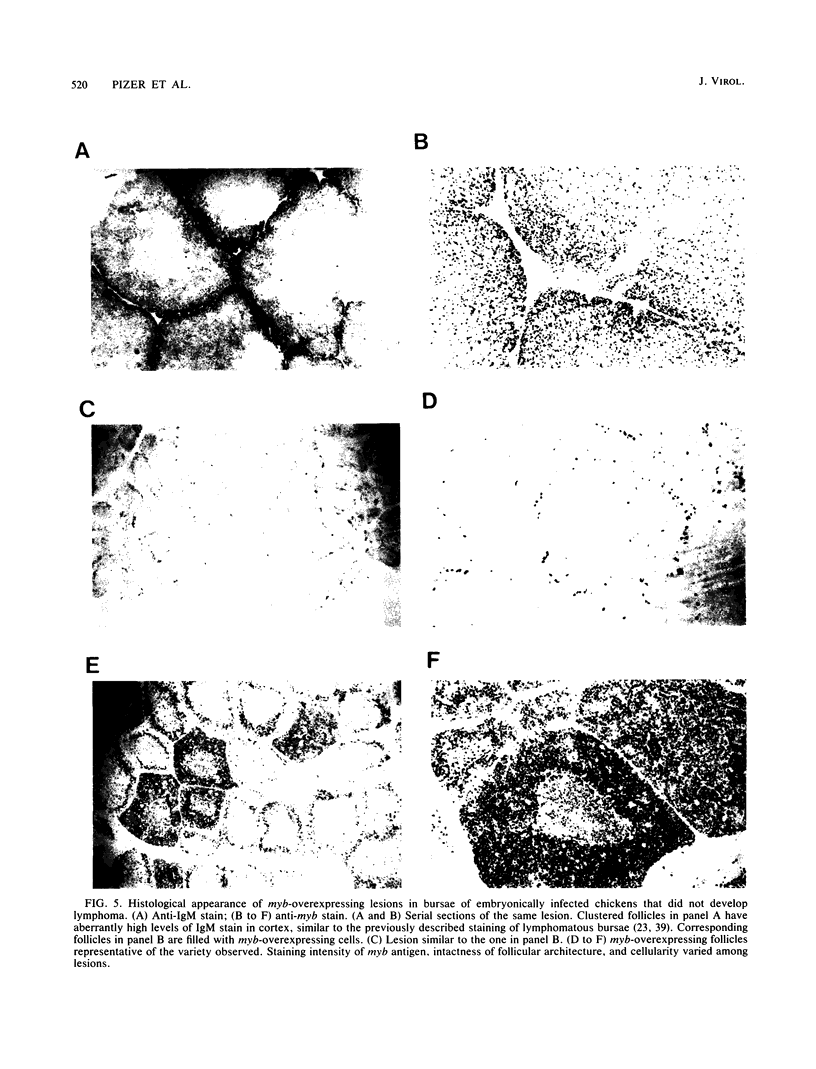
We have previously reported that infection of 9- to 13-day-old chicken embryos with RAV-1 results in rapid development of a novel B-cell lymphoma in which proviral insertion has activated expression of the c-myb gene (E. Pizer and E. H. Humphries, J. Virol. 63:1630-1640, 1989). The biological properties of these B-cell lymphomas are distinct from those associated with the B-cell lymphomas that develop following avian leukosis virus proviral insertion within the c-myc locus. In an extension of this study, more than 200 chickens, infected as 10- to 11-day-old embryos, were examined for development of lymphomas that possess disrupted c-myb loci. Fourteen percent developed disseminated B-cell lymphoma. In the majority of these tumors, the RAV-1 provirus had inserted between the first and second exons that code for p75c-myb. However, insertions between the second and third exons and between the third and fourth exons were also detected. In situ analysis of myb protein expression in tumor tissue revealed morphological features suggesting that the tumor originates in the bursa. Within the bursa, the lymphoma appeared to spread from follicle to follicle without compromising the structural integrity of the organ. Tumor masses in liver demonstrated heterogeneous levels of myb protein suggestive of biologically distinct subpopulations. In contrast to the morbidity data, immunohistological analysis of bursae from 4- to 6-week-old chickens at risk of developing lymphomas bearing altered c-myb loci revealed lesions expressing elevated levels of myb in 16 of 19 birds. The activated myb lymphoma displayed very poor capacity to proliferate outside its original host. Only 1 of 33 in vivo transfers of tumor to recipient hosts established a transplantable tumor. None of the primary tumor tissue nor the transplantable tumor exhibited the capacity for in vitro proliferation. Similar experimental manipulation has yielded in vitro lines established from avian B-cell lymphomas expressing elevated levels of c-myc or v-rel. The dependence on embryonic infection for development of activated-myb lymphoma suggests a requirement for a specific target cell in which c-myb is activated by proviral insertion. It is likely, moreover, that continued tumor development requires elevated expression of myb proteins within a specific cell population in a restricted stage of differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba T. W., Giroir B. P., Humphries E. H. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology. 1985 Jul 15;144(1):139–151. doi: 10.1016/0042-6822(85)90312-5. [DOI] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Avian leukosis virus infection: analysis of viremia and DNA integration in susceptible and resistant chicken lines. J Virol. 1984 Jul;51(1):123–130. doi: 10.1128/jvi.51.1.123-130.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba T. W., Humphries E. H. Formation of a transformed follicle is necessary but not sufficient for development of an avian leukosis virus-induced lymphoma. Proc Natl Acad Sci U S A. 1985 Jan;82(1):213–216. doi: 10.1073/pnas.82.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C. F., Humphries E. H. A nonimmunosuppressive helper virus allows high efficiency induction of B cell lymphomas by reticuloendotheliosis virus strain T. J Exp Med. 1988 Jan 1;167(1):89–108. doi: 10.1084/jem.167.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender T. P., Kuehl W. M. Differential expression of the c-myb proto-oncogene marks the pre-B cell/B cell junction in murine B lymphoid tumors. J Immunol. 1987 Dec 1;139(11):3822–3827. [PubMed] [Google Scholar]
- Bender T. P., Thompson C. B., Kuehl W. M. Differential expression of c-myb mRNA in murine B lymphomas by a block to transcription elongation. Science. 1987 Sep 18;237(4821):1473–1476. doi: 10.1126/science.3498214. [DOI] [PubMed] [Google Scholar]
- Beug H., Blundell P. A., Graf T. Reversibility of differentiation and proliferative capacity in avian myelomonocytic cells transformed by tsE26 leukemia virus. Genes Dev. 1987 May;1(3):277–286. doi: 10.1101/gad.1.3.277. [DOI] [PubMed] [Google Scholar]
- Beug H., Leutz A., Kahn P., Graf T. Ts mutants of E26 leukemia virus allow transformed myeloblasts, but not erythroblasts or fibroblasts, to differentiate at the nonpermissive temperature. Cell. 1984 Dec;39(3 Pt 2):579–588. doi: 10.1016/0092-8674(84)90465-3. [DOI] [PubMed] [Google Scholar]
- Biedenkapp H., Borgmeyer U., Sippel A. E., Klempnauer K. H. Viral myb oncogene encodes a sequence-specific DNA-binding activity. Nature. 1988 Oct 27;335(6193):835–837. doi: 10.1038/335835a0. [DOI] [PubMed] [Google Scholar]
- Chen J. H. Expression of endogenous avian myeloblastosis virus information in different chicken cells. J Virol. 1980 Oct;36(1):162–170. doi: 10.1128/jvi.36.1.162-170.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clurman B. E., Hayward W. S. Multiple proto-oncogene activations in avian leukosis virus-induced lymphomas: evidence for stage-specific events. Mol Cell Biol. 1989 Jun;9(6):2657–2664. doi: 10.1128/mcb.9.6.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Payne L. N., Dent P. B., Burmester B. R., Good R. A. Pathogenesis of avian lymphoid leukosis. I. Histogenesis. J Natl Cancer Inst. 1968 Aug;41(2):373–378. [PubMed] [Google Scholar]
- Dvorák M., Trávnícek M., Sulová A., Ríman J. Two exons specific for the myb proto-oncogene found upstream from the avian myeloblastosis virus-transduced myb sequences. Folia Biol (Praha) 1987;33(1):1–10. [PubMed] [Google Scholar]
- Eskola J., Toivanen P. Effect of in ovo treatment with cyclophosphamide on lymphoid system in chicken. Cell Immunol. 1974 Sep;13(3):459–471. doi: 10.1016/0008-8749(74)90265-2. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Bishop J. M. Isolation of monoclonal antibodies specific for products of avian oncogene myb. Mol Cell Biol. 1984 Dec;4(12):2843–2850. doi: 10.1128/mcb.4.12.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Bishop J. M. Structure and transcription of the cellular homolog (c-myb) of the avian myeloblastosis virus transforming gene (v-myb). J Virol. 1983 Apr;46(1):212–220. doi: 10.1128/jvi.46.1.212-220.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Ramsay R. G., Johnson G. R. Murine myeloid cell lines derived by in vitro infection with recombinant c-myb retroviruses express myb from rearranged vector proviruses. EMBO J. 1989 Jun;8(6):1767–1775. doi: 10.1002/j.1460-2075.1989.tb03570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S. L., Hahn M., Hayward W. S. Structural organization of upstream exons and distribution of transcription start sites in the chicken c-myb gene. Mol Cell Biol. 1989 Feb;9(2):837–843. doi: 10.1128/mcb.9.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Kanter M. R., Smith R. E., Hayward W. S. Rapid induction of B-cell lymphomas: insertional activation of c-myb by avian leukosis virus. J Virol. 1988 Apr;62(4):1423–1432. doi: 10.1128/jvi.62.4.1423-1432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Bishop J. M. Neoplastic transformation by E26 leukemia virus is mediated by a single protein containing domains of gag and myb genes. J Virol. 1984 Apr;50(1):280–283. doi: 10.1128/jvi.50.1.280-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempnauer K. H., Gonda T. J., Bishop J. M. Nucleotide sequence of the retroviral leukemia gene v-myb and its cellular progenitor c-myb: the architecture of a transduced oncogene. Cell. 1982 Dec;31(2 Pt 1):453–463. doi: 10.1016/0092-8674(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. The highly conserved amino-terminal region of the protein encoded by the v-myb oncogene functions as a DNA-binding domain. EMBO J. 1987 Sep;6(9):2719–2725. doi: 10.1002/j.1460-2075.1987.tb02565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin N. M., Houssaint E., Jotereau F. Differentiation of the primary lymphoid organs in avian embryos: origin and homing of the lymphoid stem cells. Adv Exp Med Biol. 1977;88:29–37. doi: 10.1007/978-1-4613-4169-7_3. [DOI] [PubMed] [Google Scholar]
- Moscovici M. G., Moscovici C. Isolation and characterization of a temperature-sensitive mutant of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1421–1425. doi: 10.1073/pnas.80.5.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Gasic G. P., Rogler C. E., Skalka A. M., Ju G., Hishinuma F., Papas T., Astrin S. M., Hayward W. S. Molecular analysis of the c-myc locus in normal tissue and in avian leukosis virus-induced lymphomas. J Virol. 1982 Oct;44(1):158–166. doi: 10.1128/jvi.44.1.158-166.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Neiman P., Payne L. N., Weiss R. A. Viral DNA in bursal lymphomas induced by avian leukosis viruses. J Virol. 1980 Apr;34(1):178–186. doi: 10.1128/jvi.34.1.178-186.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman P., Wolf C., Enrietto P. J., Cooper G. M. A retroviral myc gene induces preneoplastic transformation of lymphocytes in a bursal transplantation assay. Proc Natl Acad Sci U S A. 1985 Jan;82(1):222–226. doi: 10.1073/pnas.82.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness S. A., Beug H., Graf T. v-myb dominance over v-myc in doubly transformed chick myelomonocytic cells. Cell. 1987 Oct 9;51(1):41–50. doi: 10.1016/0092-8674(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Ness S. A., Marknell A., Graf T. The v-myb oncogene product binds to and activates the promyelocyte-specific mim-1 gene. Cell. 1989 Dec 22;59(6):1115–1125. doi: 10.1016/0092-8674(89)90767-8. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Bishop J. M., Varmus H. E. Multiple arrangements of viral DNA and an activated host oncogene in bursal lymphomas. Nature. 1982 Jan 21;295(5846):209–214. doi: 10.1038/295209a0. [DOI] [PubMed] [Google Scholar]
- Perbal B., Cline J. M., Hillyard R. L., Baluda M. A. Organization of chicken DNA sequences homologous to the transforming gene of avian myeloblastosis virus. II. Isolation and characterization of lambda proto-amv DNA recombinant clones from a library of leukemic chicken DNA. J Virol. 1983 Mar;45(3):925–940. doi: 10.1128/jvi.45.3.925-940.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer E., Humphries E. H. RAV-1 insertional mutagenesis: disruption of the c-myb locus and development of avian B-cell lymphomas. J Virol. 1989 Apr;63(4):1630–1640. doi: 10.1128/jvi.63.4.1630-1640.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchase H. G., Gilmour D. G. Lymphoid leukosis in chickens chemically bursectomized and subsequently inoculated with bursa cells. J Natl Cancer Inst. 1975 Oct;55(4):851–855. doi: 10.1093/jnci/55.4.851. [DOI] [PubMed] [Google Scholar]
- Ratcliffe M. J., Lassila O., Reynolds J., Pink J. R., Vainio O. A re-evaluation of the function of the bursa of Fabricius. Prog Clin Biol Res. 1987;238:3–14. [PubMed] [Google Scholar]
- Shen-Ong G. L., Morse H. C., 3rd, Potter M., Mushinski J. F. Two modes of c-myb activation in virus-induced mouse myeloid tumors. Mol Cell Biol. 1986 Feb;6(2):380–392. doi: 10.1128/mcb.6.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Expression of the c-myb proto-oncogene during cellular proliferation. 1986 Jan 30-Feb 5Nature. 319(6052):374–380. doi: 10.1038/319374a0. [DOI] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A., Lassila O., Pink J. R. Rearrangement of chicken immunoglobulin genes is not an ongoing process in the embryonic bursa of Fabricius. Proc Natl Acad Sci U S A. 1986 May;83(10):3336–3340. doi: 10.1073/pnas.83.10.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill J. C., Reynaud C. A. The chicken B cell compartment. Science. 1987 Nov 20;238(4830):1094–1098. doi: 10.1126/science.3317827. [DOI] [PubMed] [Google Scholar]
- Weston K., Bishop J. M. Transcriptional activation by the v-myb oncogene and its cellular progenitor, c-myb. Cell. 1989 Jul 14;58(1):85–93. doi: 10.1016/0092-8674(89)90405-4. [DOI] [PubMed] [Google Scholar]