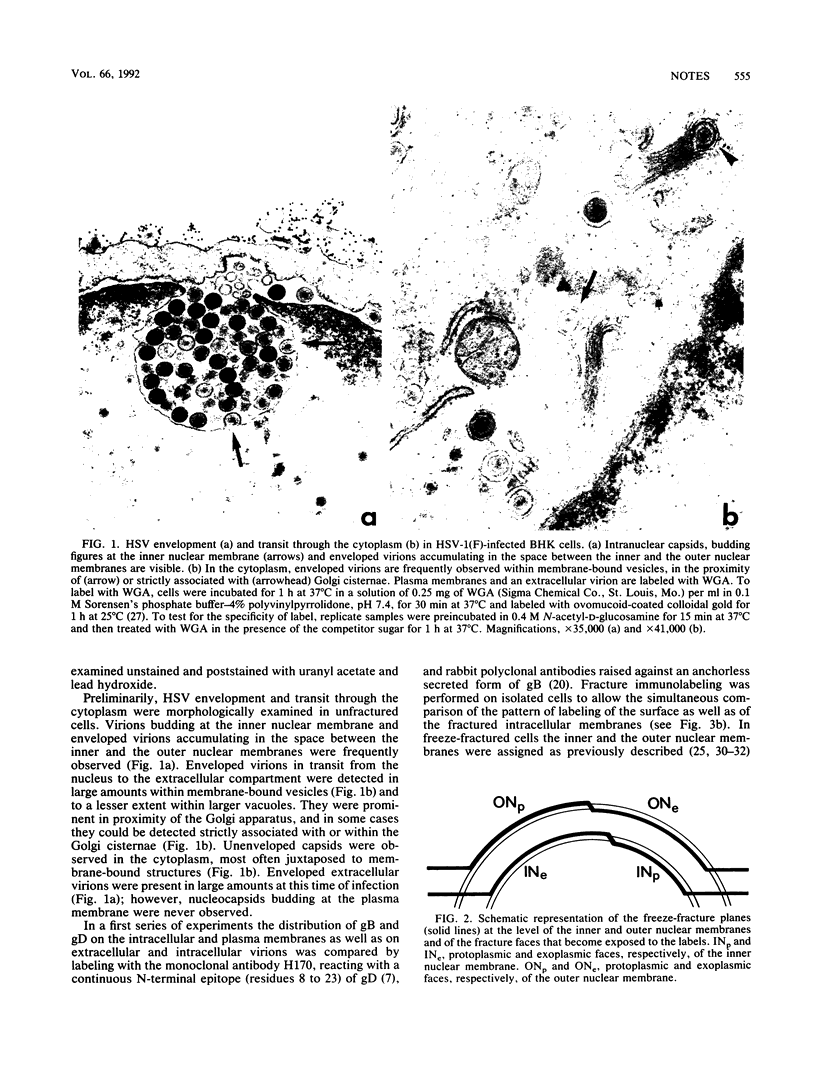
Abstract
Herpes simplex virus envelopment and maturation were investigated by thin-section fracture label. The distribution of glycoproteins B and D was analyzed by labeling with antibodies; the precursor and mature forms of the glycoproteins were differentiated by labeling with the lectins concanavalin A (ConA) and wheat germ agglutinin (WGA), respectively. We report that the two glycoproteins were readily detected in the intracellular virion, whether located between the inner and outer nuclear membranes or within cytoplasmic membrane-bound vesicles and in the inner and outer nuclear membranes themselves. The enveloped virion between the inner and outer nuclear membranes labeled with ConA but not with WGA. During the transit to the extracellular space the reactivity of the virion membranes with ConA decreased and that with WGA ensued. The results document that herpes simplex viruses acquire at the inner nuclear membrane an envelope carrying the immature forms of the glycoproteins and that during the transit to the extracellular space the envelope glycoproteins become of the fully processed type.
Full text
PDF
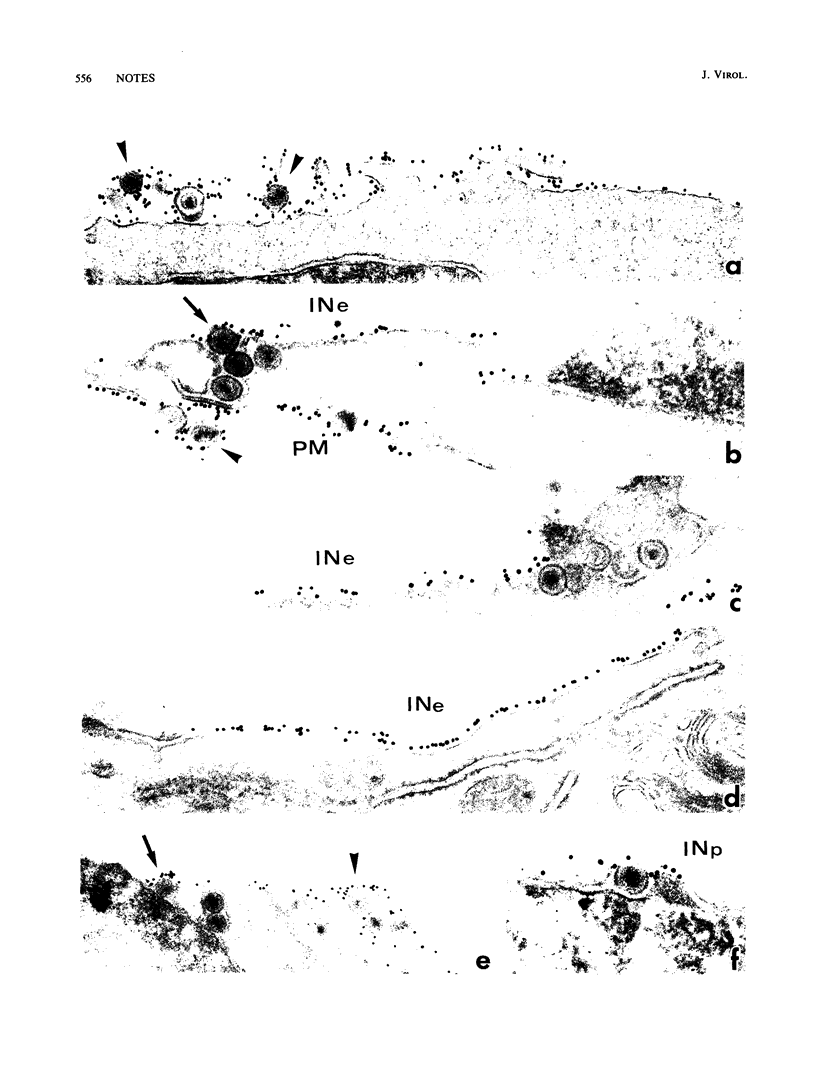

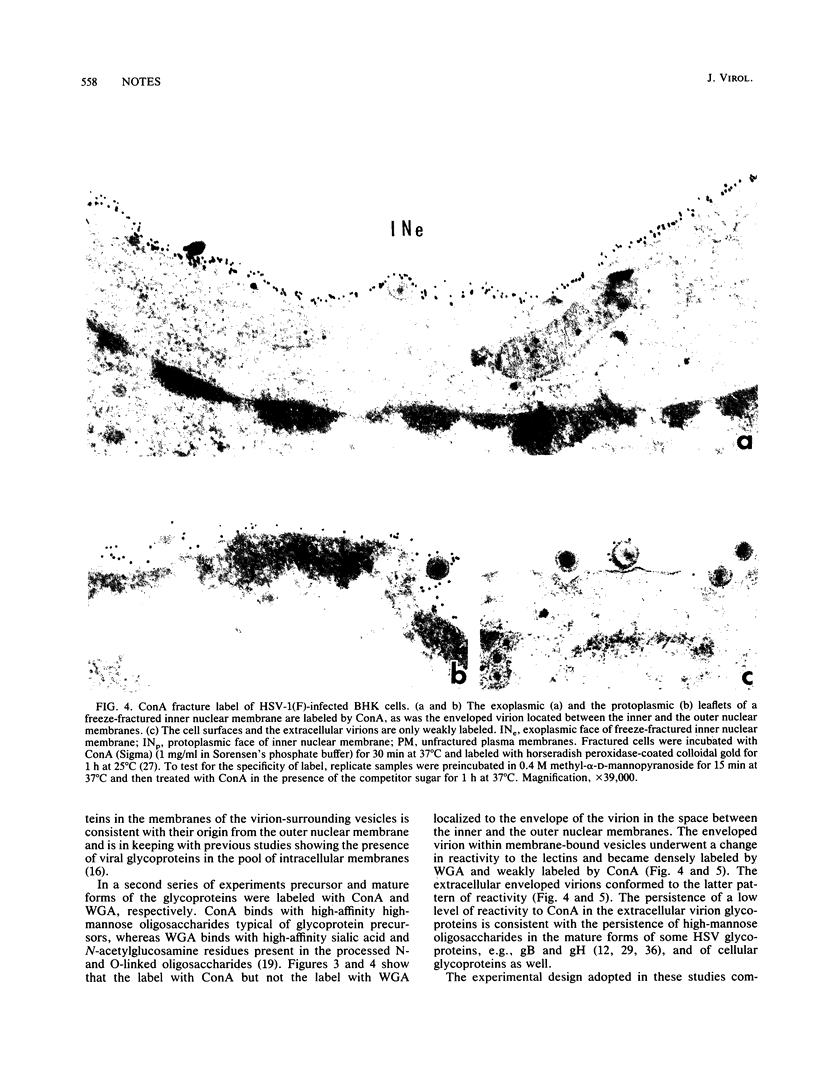
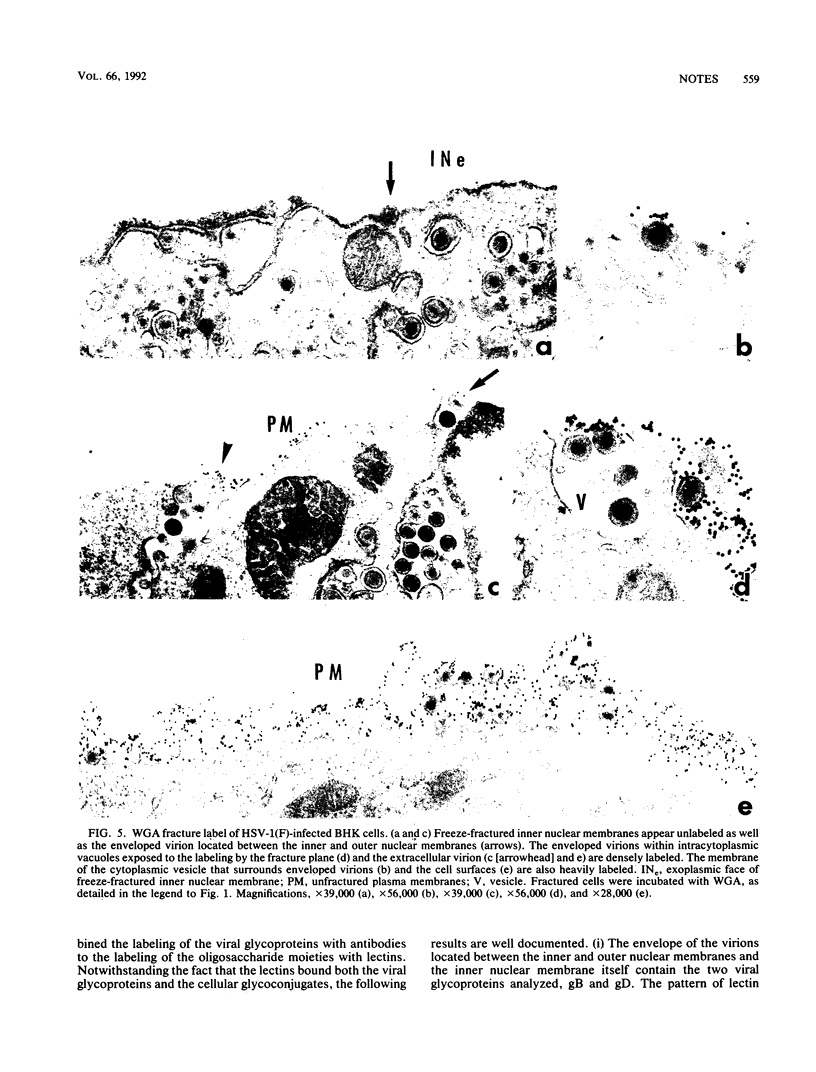
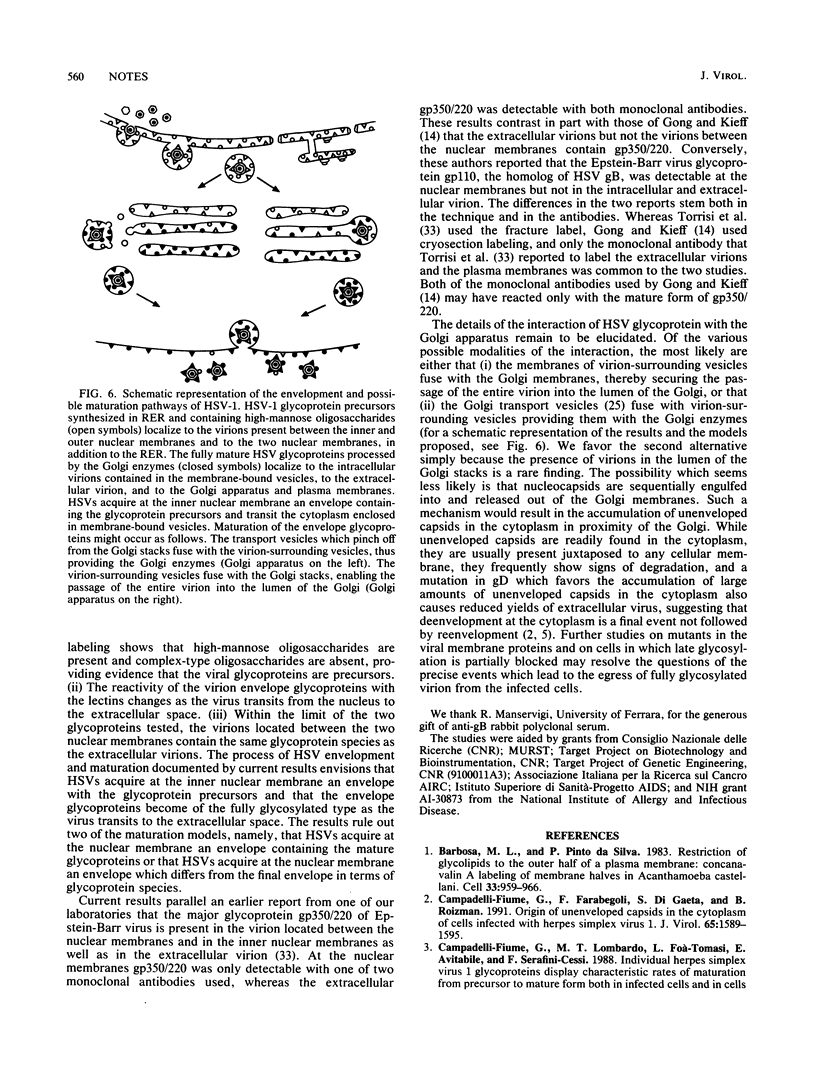

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa M. L., Pinto da Silva P. Restriction of glycolipids to the outer half of a plasma membrane: concanavalin A labeling of membrane halves in Acanthamoeba castellanii. Cell. 1983 Jul;33(3):959–966. doi: 10.1016/0092-8674(83)90039-9. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Farabegoli F., Di Gaeta S., Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991 Mar;65(3):1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Lombardo M. T., Foà-Tomasi L., Avitabile E., Serafini-Cessi F. Individual herpes simplex virus 1 glycoproteins display characteristic rates of maturation from precursor to mature form both in infected cells and in cells that constitutively express the glycoproteins. Virus Res. 1988 Apr;10(1):29–40. doi: 10.1016/0168-1702(88)90055-x. [DOI] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Poletti L., Dall'Olio F., Serafini-Cessi F. Infectivity and glycoprotein processing of herpes simplex virus type 1 grown in a ricin-resistant cell line deficient in N-acetylglucosaminyl transferase I. J Virol. 1982 Sep;43(3):1061–1071. doi: 10.1128/jvi.43.3.1061-1071.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Qi S., Avitabile E., Foà-Tomasi L., Brandimarti R., Roizman B. Glycoprotein D of herpes simplex virus encodes a domain which precludes penetration of cells expressing the glycoprotein by superinfecting herpes simplex virus. J Virol. 1990 Dec;64(12):6070–6079. doi: 10.1128/jvi.64.12.6070-6079.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- FALKE D., SIEGERT R., VOGELL W. [Electron microscopic findings on the problem of double membrane formation in herpes simplex virus]. Arch Gesamte Virusforsch. 1959;9:484–496. [PubMed] [Google Scholar]
- Foà-Tomasi L., Avitabile E., Boscaro A., Brandimarti R., Gualandri R., Manservigi R., Dall'Olio F., Serafini-Cessi F., Fiume G. C. Herpes simplex virus (HSV) glycoprotein H is partially processed in a cell line that expresses the glycoprotein and fully processed in cells infected with deletion or ts mutants in the known HSV glycoproteins. Virology. 1991 Feb;180(2):474–482. doi: 10.1016/0042-6822(91)90061-f. [DOI] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Gong M., Kieff E. Intracellular trafficking of two major Epstein-Barr virus glycoproteins, gp350/220 and gp110. J Virol. 1990 Apr;64(4):1507–1516. doi: 10.1128/jvi.64.4.1507-1516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine J. W., Spear P. G., Roizman B. Proteins specified by herpes simplex virus. VI. Viral proteins in the plasma membrane. J Virol. 1972 Mar;9(3):431–439. doi: 10.1128/jvi.9.3.431-439.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J Virol. 1982 Sep;43(3):1102–1112. doi: 10.1128/jvi.43.3.1102-1112.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousoulas K. G., Bzik D. J., DeLuca N., Person S. The effect of ammonium chloride and tunicamycin on the glycoprotein content and infectivity of herpes simplex virus type 1. Virology. 1983 Mar;125(2):468–474. doi: 10.1016/0042-6822(83)90217-9. [DOI] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses as observed in the electron microscope. I. Herpes simplex virus. J Exp Med. 1954 Aug 1;100(2):195–202. doi: 10.1084/jem.100.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ROSE H. M., HOLDEN M., JONES E. P. Electron microscopic observations on the development of herpes simplex virus. J Exp Med. 1959 Oct 1;110:643–656. doi: 10.1084/jem.110.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manservigi R., Grossi M. P., Gualandri R., Balboni P. G., Marchini A., Rotola A., Rimessi P., Di Luca D., Cassai E., Barbanti-Brodano G. Protection from herpes simplex virus type 1 lethal and latent infections by secreted recombinant glycoprotein B constitutively expressed in human cells with a BK virus episomal vector. J Virol. 1990 Jan;64(1):431–436. doi: 10.1128/jvi.64.1.431-436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Qadri I., Navarro D., Gimeno C. Antigenic and structural properties of mutants in herpes simplex virus 1 glycoprotein B. Adv Exp Med Biol. 1990;278:165–182. doi: 10.1007/978-1-4684-5853-4_17. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Torrisi M. R., Kachar B. Freeze-fracture cytochemistry: localization of wheat-germ agglutinin and concanavalin A binding sites on freeze-fractured pancreatic cells. J Cell Biol. 1981 Nov;91(2 Pt 1):361–372. doi: 10.1083/jcb.91.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Campadelli-Fiume G. Studies on benzhydrazone, a specific inhibitor of herpesvirus glycoprotein synthesis. Size distribution of glycopeptides and endo-beta-N-acetylglucosaminidase-H treatment. Arch Virol. 1981;70(4):331–343. doi: 10.1007/BF01320248. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Scannavini M., Campadelli-Fiume G. Processing of herpes simplex virus-1 glycans in cells defective in glycosyl transferases of the Golgi system: relationship to cell fusion and virion egress. Virology. 1983 Nov;131(1):59–70. doi: 10.1016/0042-6822(83)90533-0. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Bonatti S. Immunocytochemical study of the partition and distribution of Sindbis virus glycoproteins in freeze-fractured membranes of infected baby hamster kidney cells. J Cell Biol. 1985 Oct;101(4):1300–1306. doi: 10.1083/jcb.101.4.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Cirone M., Pavan A., Zompetta C., Barile G., Frati L., Faggioni A. Localization of Epstein-Barr virus envelope glycoproteins on the inner nuclear membrane of virus-producing cells. J Virol. 1989 Feb;63(2):828–832. doi: 10.1128/jvi.63.2.828-832.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Da Silva P. P. T-lymphocyte heterogeneity: wheat germ agglutinin labeling of transmembrane glycoproteins. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5671–5674. doi: 10.1073/pnas.79.18.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrisi M. R., Lotti L. V., Pavan A., Migliaccio G., Bonatti S. Free diffusion to and from the inner nuclear membrane of newly synthesized plasma membrane glycoproteins. J Cell Biol. 1987 Mar;104(3):733–737. doi: 10.1083/jcb.104.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva P. P., Parkison C., Dwyer N. Freeze-fracture cytochemistry: thin sections of cells and tissues after labeling of fractures faces. J Histochem Cytochem. 1981 Aug;29(8):917–928. doi: 10.1177/29.8.7276536. [DOI] [PubMed] [Google Scholar]