Figure 1.
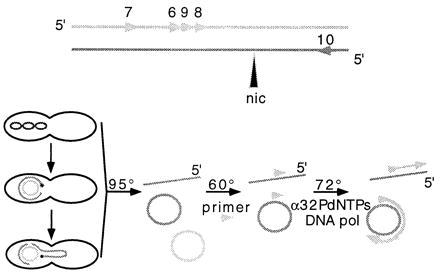
Diagram of the nicking assay. The reaction is a runoff DNA synthesis with a thermostable DNA polymerase using as template ss linear or ss circular molecules, released from lysed bacteria, which have been hybridized to a single primer (triangle) 100–250 bases 3′ to the 5′ end of a suspected nick site. DNA synthesis proceeds (Upper, top strand) until the polymerase encounters an interruption (nic) in the DNA template (nicked substrates) or reaches the limits of its processivity (unnicked molecules). These unspecifically terminated reaction products can be distinguished by size from the much smaller specific termination products using a sequencing gel. The source of the specific DNA cleavage event in vivo could be conjugative transfer of a plasmid, as depicted (Lower). Cell lysis and denaturation of the released double-stranded DNA molecules (95°C), hybridization of one specific complementary DNA primer (60°C), and synthesis on primed DNA templates (72°C) are achieved in three successive incubations of the reaction mixture for 35 cycles in a thermocycler. The reaction products are labeled with [α-32P]dNTP precursors. (Upper) Primers 6–9, complementary to the sequences of the T strand and primer 10, complementary to the retained strand, within the oriT of F-like plasmids.
