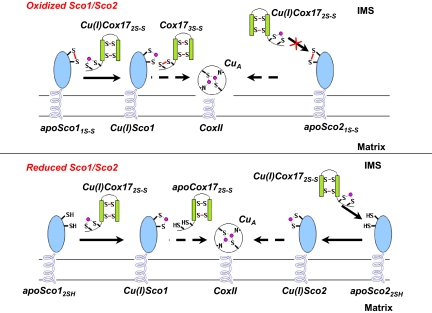
Fig. 4.
Proposed mechanism of copper transfer from HCox172S-S to the subunit II of CcO, through the assistance of HSco1 and HSco2. This model implies that HSco1, independently of the redox state of its Cys ligands in the IMS, may accept copper(I) from the mitochondrial chaperone Cu(I)HCox172S-S to form Cu(I)HSco1 whereas HSco2 can accept copper(I) from Cu(I)HCox172S-S only once its Cys ligands in the IMS are in a reduced state. HCox173S-S, produced by the redox HSco1/HCox172S-S reaction, can be quickly reduced to HCox172S-S by GSH, thus the latter protein being recycled for the following metal transfer.