Fig. 5.
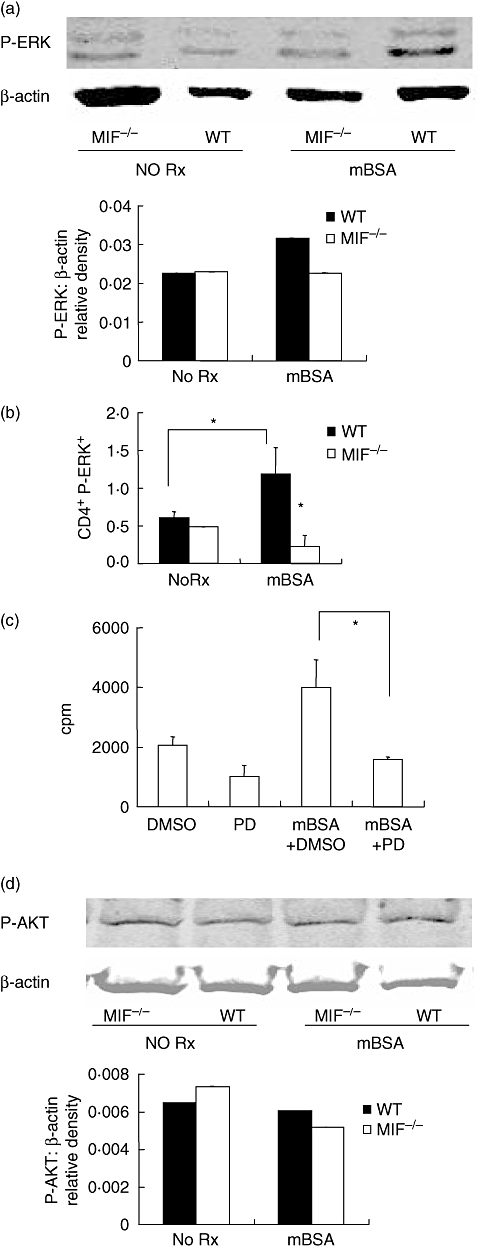
Analysis of phosphorylated-extracellular signal-regulated kinase (P-ERK) and serine/threonine kinase (AKT). Splenocytes were cultured for 6 h with and without methylated bovine serum albumin (mBSA). Phospho-ERK and phospho-AKT were measured by Western blotting and the membranes reprobed for β-actin as loading control. Blots are representative of three independent experiments. Migration inhibitory factor (MIF) −/− cells had decreased P-ERK compared with wildtype (WT) mice following mBSA stimulation (a). To identify the lymphocyte population expressing P-ERK, splenocytes were simultaneously labelled with CD4-phycoerythrin and P-ERK-fluorescein isothiocyanate for flow cytometric analysis. Results are expressed as percent positive for P-ERK in gated population and are data from a representative of nine experiments (b). Stimulation with mBSA resulted in significantly increased P-ERK in WT CD4+. MIF −/− cells did not exhibit an increase in P-ERK in response to mBSA. Inhibition of ERK1/2 by 30 min pretreatment with PD98059 inhibited antigen-stimulated cell proliferation (c) (*P < 0·05). DMSO control pretreatment had no inhibitory effect. Western blotting for P-AKT shows comparable basal and antigen-stimulated P-AKT in both WT and MIF −/− cells (d). Antigen-stimulation did not increase P-AKT in either group (d).
