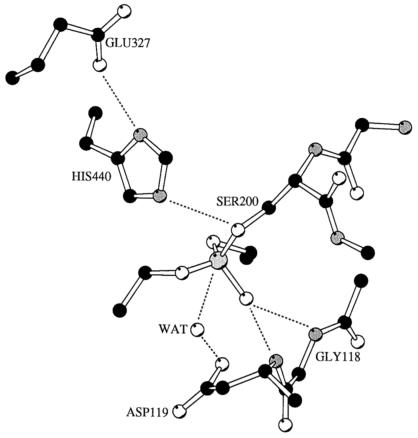
Figure 3.
Model of the active site region of AChE (39) with Gly119 (Gly137 in E3) substituted with an aspartate. Catalytic triad residues include Glu327, His440, and Ser200. Dashed lines indicate the positions of possible hydrogen bonds. A diethyl phosphate moiety has been attached to Ser200 to show the covalent intermediate that forms by the reaction of an OP with an esterase, and the position of a hypothetical water molecule (WAT) is indicated. The aspartate residue may act as a general base that enables water to attack the phosphorus atom, resulting in cleavage of the bond with Ser200. The direction of attack by the water molecule must be nearly in line with the bond to be cleaved (47, 48), and the model indicates that this is possible. The phosphoryl oxygen has been oriented toward the main chain nitrogen atoms of residues Gly118 and Asp119. Hydrogen bonds with these atoms may stabilize the oxyanion that would form during hydrolysis of the bond between Ser200 and the phosphorus atom. Modeling of the position of the water and Asp119 was conducted using van der Waals limitations and usual bond angles, and the model was rendered using molscript (40).