Figure 6.
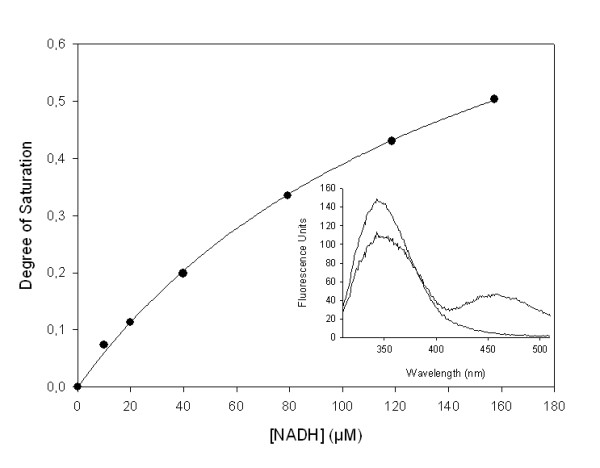
Equilibrium binding of NADH to MtCS assessed by monitoring the protein fluorescence quench upon binary complex formation. The binding of NADH to MtCS causes a quench in protein fluorescence (λexc = 290 nm; 310 ≤ λem ≤ 510 nm; with a maximum at 345.5 nm). The MtCS enzyme solution (1 μM) was titrated with increasing concentrations of NADH, and the data points (fluorescence intensities at 345.5 nm) were fitted to a hyperbolic equation (solid line). Inset: Emission spectra of free MtCS (1 μM) and enzyme in the presence of 80 μM NADH. Emission spectrum of free enzyme shows a peak at 345.5 nm and no emission at ~450 nm. Emission spectrum of MtCS in the presence of NADH shows a quench in protein fluorescence concomitant to an increase in nucleotide fluorescence at ~450 nm.
