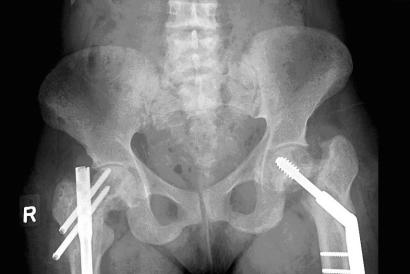
Case report
In March 1999, a 49-year-old woman presented with acute right hip pain and no history of trauma. She had been diagnosed with rapidly progressive glomerulonephritis in 1966 leading to complete renal failure in 1973. Treatment included multiple failed attempts at transplantation and long-term hemodialysis. She was diagnosed with beta-2 microglobulin amyloidosis in 1986. She had a para-thyroidectomy for secondary hyperparathyroidism, a history of renal osteo-dystrophy, multiple surgical releases and tenosynovectomies for bilateral carpal tunnel syndrome and trigger finger, and polyarthritis. Radiographs demonstrated a large lytic defect in the right femoral neck. A smaller lytic defect was noted in the left femoral head. As prophylaxis, the patient underwent internal fixation of the right hip with a dynamic hip screw (DHS). Curetted bone taken from the lesion at the time of surgery stained positive with Congo red and exhibited the characteristic dichroism of amyloid. Immunoperoxidase stains for beta-2 microglobulin were also positive.
Six months postoperatively, hip discomfort persisted and the patient remained unable to fully weight-bear. Despite a well-fixed pin and plate, the large lytic defect in the right femoral neck was unresolved, and no signs of healing were evident on radiography. In addition, she exhibited diffuse myofascial pain and muscle spasms. This pain was distinct from her bony pain and was especially prevalent in her pelvic girdle. Physiotherapy as well as narcotics, mexiletine, benzodiazepines and systemic xylocaine were tried with some success. Selective serotonin reuptake inhibitors (SSRIs) and antiepileptics were used for a painful femoral neuropathy.
In June 2002, the patient developed severe left hip pain, and radiographs revealed an incomplete fracture through the previously identified lytic defect. Open reduction and internal fixation with a DHS was performed. In April 2003, the patient experienced a further increase of her longstanding right hip pain. Radiographically, marked angulation at the inferior aspect of the prosthesis was seen and was attributed to fracture of the proximal femoral shaft. The DHS was removed, and revision with a locked femoral antegrade intramedullary nail was undertaken. Biopsy showed no evidence of amyloid at this fracture site. Investigation of severe right thigh pain postoperatively revealed an intramuscular amyloid deposit, palpable on physical examination. Radiographs in January 2004 showed evidence of healing around the right femoral shaft fracture. Fracture lines at the left hip did not show signs of healing, but at the right hip, the fracture lines showed early callous. See Figure 1 and Figure 2 for radiographs.
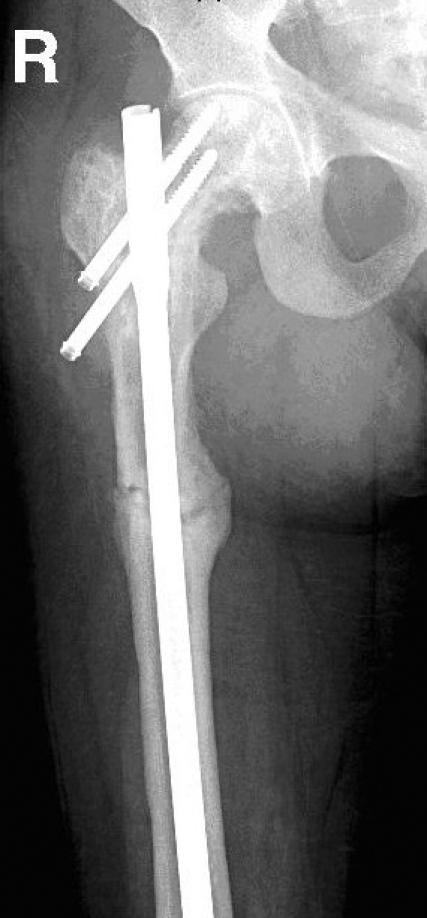
FIG. 1. Anteroposterior view of right proximal femur, taken in February 2005.
FIG. 2. Anteroposterior view of pelvis, taken in February 2005.
Discussion
Dialysis-related amyloidosis (DRA) is a serious complication of both peritoneal dialysis and hemodialysis, being almost universal among patients on dialysis for 15 years or more.1 Beta-2 microglobulin deposits preferentially in bone and synovium but in other tissues as well, including tendons and peripheral nerves. Complications such as cystic bone lesions, carpal tunnel syndrome, spondyloarthro-pathy, arthritis and periarthritis, spinal canal stenosis, and systemic organ involvement are increasing in incidence as the survival of patients on long-term dialysis improves.2,3
DRA bone lesions differ from renal osteodystrophy in that the presence of the amyloid deposits interfere with normal bone tissue dynamics by processes yet to be fully understood. In renal osteodystrophy, the abnormality is in the underlying metabolic process itself, causing bone turnover to be either increased or decreased owing to systemic imbalances of elements such as phosphorus and aluminum.4 In the case of pathological fractures in dialysis patients, it is essential that DRA be distinguished from renal osteodystrophy because there are implications for healing. Theoretically, if the interference from the beta-2 microglobulin deposits of DRA could be eliminated, or the healing process could be enhanced (such as with bone grafting), relatively normal processes could proceed with callous formation. Conversely, in renal osteodystrophy, the underlying metabolic disruption makes normal callous formation less likely with interventions targeted solely at the level of the bone. DRA can only be definitively diagnosed with a tissue sample containing documented beta-2 micro-globulin.3
Kidney transplantation is currently the only way to prevent progression of DRA. Experimental beta-2 microglobulin adsorption columns, used as an adjunct to dialysis, show some promise. Until effective and practical means to prevent and reverse amyloid deposition are achieved, the current practice is to treat symptomatically. Resultant or impending fractures are managed in younger, ambulatory patients with traditional orthopedic implants. Carpal tunnel syndrome and spinal stenosis are also managed with surgery, and pain secondary to other tissue deposition is treated with typical pharmacological analgesia, antiinflammatories and physical therapy.2,5 There are no unique interventions reported for the palliation of DRA-associated pain syndromes.
Orthopedic interventions have high failure rates in DRA compared with the general population. Interference of amyloid with connective tissue metabolism, mechanical weakness secondary to the physical presence of the deposits and stimulation of a destructive inflammatory process are all theories for this failure. Nonunion, aseptic loosening of hardware and further fractures are commonplace, leading frequently to the need for early revision.5 In some cases, curettage of cystic lesions followed by bone grafting has been used as an adjunct to operative repair in an attempt to reduce the incidence of such complications.6 Internal fixation was beneficial in this case, providing pain relief as well as a relatively satisfactory degree of postoperative ambulation. Additionally, some healing was noted — something that seems to be fairly uncommon due to the predominantly osteoclastic nature of DRA.1 However, pain due to extraosseous amyloid deposition continues to cause severe disability for this patient.
Treatment of DRA-associated pain is very difficult, and the potential capacity of these patients to achieve bone healing through their lytic defects is unclear. Orthopedic surgical interventions remain the standard of care even with a typically rapid progression to failure. Bone grafting and bone graft substitutes may augment healing but do not alter the disease process. As the incidence of DRA rises with the increasing longevity of dialysis patients, attempts at improving the management of the painful and debilitating sequelae will need to be made.
Competing interests: None declared.
Accepted for publication Oct. 10, 2005
Correspondence to: Dr. Adrienne Kelly, 305-201 Walter Havill Dr., Halifax NS B3N 3J4; akelly2006@meds.uwo.ca
References
- 1.Tran M, Rutecki G, Sprague S. The pathogenesis of β2-microglobulin-indued bone lesions in dialysis-related amyloidosis. Semin Dial 2001;14:131-3. [DOI] [PubMed]
- 2.Niwa T. Dialysis-related amyloidosis: Pathogenesis focusing on AGE modification. Semin Dial 2001;14:123-6. [DOI] [PubMed]
- 3.Cronin RE, Henrich WL, Berns JS. Dialysis-related amyloidosis. [Web site of UpToDate]. Available: http://patients.uptodate.com/topic.asp?file=dialysis/8834&title=Dialysis+related+amyloidosis (accessed 2007 July 9).
- 4.Henrich WL. Pathogenesis of renal osteodystrophy. [Web site of UpToDate]. Available: http://patients.uptodate.com/topic.asp?file=dialysis/44315 (accessed 2007 July 9).
- 5.Crawford R, Athanasou N. β2-microglobulin amyloid deposition in hip revision arthroplasty tissues. Histopathology 1998;33:479-84. [DOI] [PubMed]
- 6.Shiota E, Yamaoka K, Kawano O, et al. Surgical treatments for orthopaedic complications in long-term haemodialysis patients—a review of 546 cases over the last 8 years. Fukuoka Igaku Zasshi 1998;89:261-76. [PubMed]