FIGURE 5. Bcl-2 specifically binds to GSH in vitro, and this interaction is antagonized by BH3 mimetics.
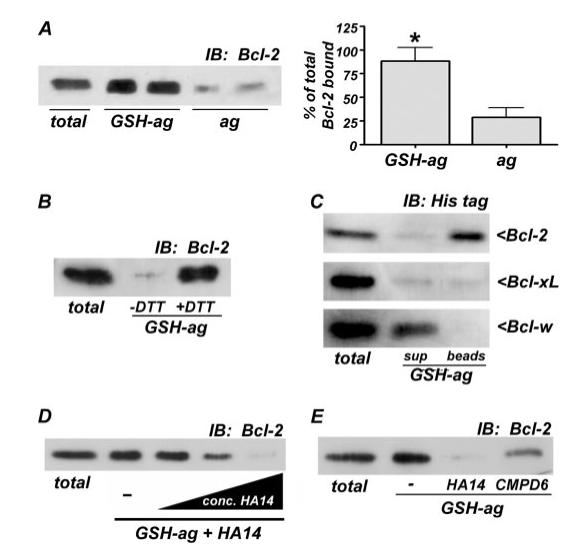
A, agarose beads conjugated to GSH (GSH-ag) but not unconjugated agarose beads alone (ag), bound to recombinant, His-tagged Bcl-2 in an in vitro pull down assay (left), as described under “Experimental Procedures.” Total His-Bcl-2 (50 ng) added to the assay is shown, and the fraction of the total Bcl-2 bound to GSH-ag or ag was quantified by densitometry (right). The data shown are from four independent experiments, each performed in duplicate. *, p < 0.01 compared with ag alone. B, the GSH-binding assay was carried out in either the absence or presence of the reducing agent, dithiothreitol (1 mm). Dithiothreitol was required for the specific interaction between Bcl-2 and GSH. C, GSH bound with higher affinity to Bcl-2 than to the homologous family members, Bcl-xL and Bcl-w. Equal amounts (50 ng) of either His-tagged Bcl-2, Bcl-xL, or Bcl-w were incubated with GSH-ag beads, followed by Western blotting of the supernatant (sup) and GSH-ag beads for the His tag. Bcl-2 bound almost entirely to GSH-ag beads (top). Although Bcl-xL bound GSH with enough affinity to be pulled out of the supernatant, the interaction was not strong enough to withstand washing, and therefore, no Bcl-xL was detected bound to the GSH-ag beads (middle). Bcl-w displayed little apparent affinity for GSH and remained primarily in the supernatant (bottom). D, His-tagged Bcl-2 binding to GSH was measured in the presence of increasing concentrations (conc.) of HA14-1 (0 (-), 100, 200, and 500 μm). The BH3 mimetic dose-dependently disrupted the in vitro interaction between Bcl-2 and GSH. E, Bcl-2 binding to GSH was assessed in the absence or presence of either of two BH3 mimetics, HA14 or CMPD6 (each at 500 μm). IB, immunoblot.
