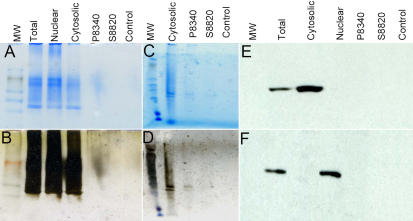
Figure 1.
Analyses of SDS–PAGE fractionated proteins in enrichment steps. A. About 100 µg of protein (initial load) was subjected to affinity enrichment on P8340 or S8820 column as indicated and total eluted and acetone precipitated proteins were subjected to SDS–PAGE fractionation and stained with Coomassie blue. An initial load (10 µg) of total, nuclear and cytosolic proteins were fractionated on a SDS–PAGE. For this purpose cytosolic and nuclear fractions (10 µg proteins each) from NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce Biotechnology Inc.) were obtained B. The same gel as in A was subjected to silver staining. C. About 250 µg of protein (initial load) was subjected to affinity enrichment on P8340 or S8820 column as indicated. Total eluted and acetone precipitated proteins were subjected to fractionation on a 4%–15% PHAST gel (GE Healthcare) and stained with Coomassie blue. D. The same gel as in C was subjected to silver staining. The control is the cytosolic fraction (4 µg) passed through an empty protein A column as described in methods. E. After transfer to a PVDF membrane, western analyses of protein extracts were performed, using GAPDH antibody and, F. Histone H3 antibody, as described in methods.