Abstract
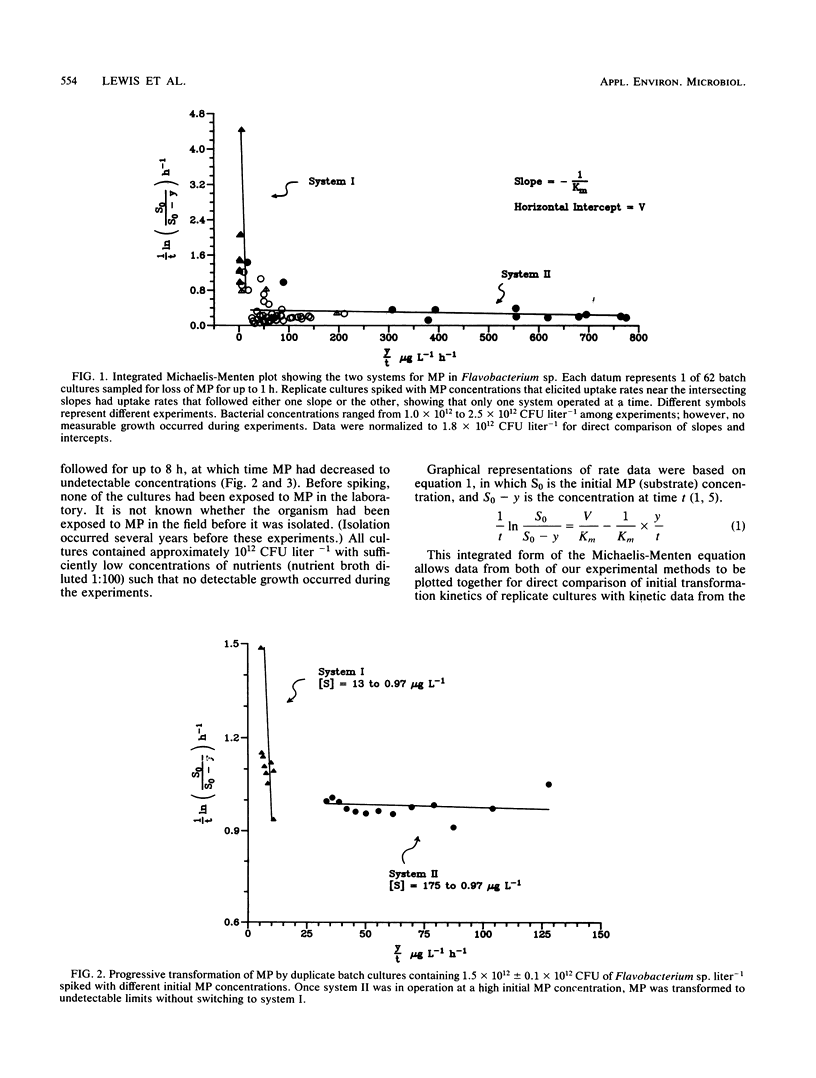
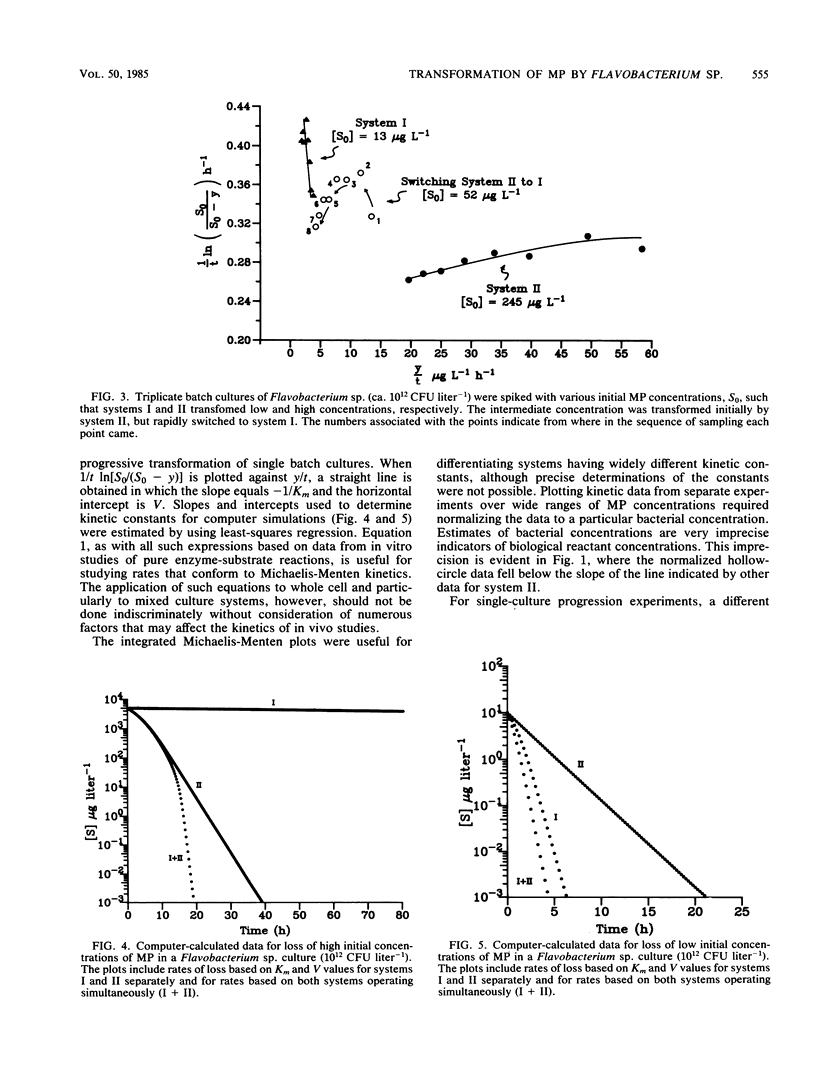
Transformation rates of an insecticide, methyl parathion, in pure cultures of Flavobacterium sp. followed multiphasic kinetics involving at least two systems (I and II). System I was a high-affinity, low-capacity system, and system II was a low-affinity, high-capacity system. Data from rate experiments suggested that metabolites formed via system II inhibited system I such that only one system operated at a time. System I operated at approximately 20 μg liter−1 and less; system II operated at approximately 4 mg liter−1 and less. These results show that xenobiotic chemicals, like naturally occurring substrates, can be transformed via multiple uptake and transformation systems even by a pure culture. Furthermore, computer simulation models of pollutant transformation rates based on kinetic constants determined in this study show that large errors can occur in predicted rates when the multiphasicity of kinetics is neglected.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L., Nimmo I. A. The reliability of Michaelis constants and maximum velocities estimated by using the integrated Michaelis-Menten equation. Biochem J. 1973 Dec;135(4):779–784. doi: 10.1042/bj1350779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew G. W., Pfaender F. K. Influence of spatial and temporal variations on organic pollutant biodegradation rates in an estuarine environment. Appl Environ Microbiol. 1983 Jan;45(1):103–109. doi: 10.1128/aem.45.1.103-109.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eighmy T. T., Bishop P. L. Multiplicity of aspartate transport in thin wastewater biofilms. Appl Environ Microbiol. 1984 Dec;48(6):1151–1158. doi: 10.1128/aem.48.6.1151-1158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Hodson R. Inhibition by peptides of amino Acid uptake by bacterial populations in natural waters: implications for the regulation of amino Acid transport and incorporation. Appl Environ Microbiol. 1984 Apr;47(4):624–631. doi: 10.1128/aem.47.4.624-631.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Hodson R. E., Freeman L. F., 3rd Effects of microbial community interactions on transformation rates of xenobiotic chemicals. Appl Environ Microbiol. 1984 Sep;48(3):561–565. doi: 10.1128/aem.48.3.561-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray R. E., Hodson R. E. Microbial biomass and utilization of dissolved organic matter in the okefenokee swamp ecosystem. Appl Environ Microbiol. 1984 Apr;47(4):685–692. doi: 10.1128/aem.47.4.685-692.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


