Abstract
To determine whether radiofrequency (RF) ablation targeting the tumor-collecting system interface has a durable effect in patients with transfusion-dependent kidney tumor-related hematuria, four patients aged 61-71 years were successfully treated with RF ablation, with a mean follow up of 12 months. Baseline creatinine levels varied from 2.0 mg/dL to 3.7 mg/dL. All patients had received red blood cell transfusions in the days and hours before RF ablation. No subsequent surgical or interventional procedures were required for management of hematuria. Gross hematuria resolved in 24-48 hours in all four patients. Two of the patients are alive with stable renal function and two died of causes unrelated to treatment. RF ablation may be an effective therapeutic option for transfusion-dependent cancer-related hematuria in patients with renal insufficiency, solitary kidney, or comorbidities, or after failed conventional therapies in patients who are not candidates for surgery.
HEMATURIA related to advanced primary or secondary renal malignancy can be a difficult problem, often complicated by renal insufficiency or comorbidities that may increase the risks and side effects of conventional therapies such as surgery or angiographic embolization. Therapeutic transcatheter embolization has been used as an effective minimally invasive option for management of intractable hematuria (1). However, this procedure can be relatively nonselective and carries the risk of contrast material-induced nephropathy and acute renal failure in patients who already have limited renal function or have undergone nephrectomy.
Radiofrequency (RF) ablation has been employed in the management of renal neoplasm-related hematuria (2). In selected patients, RF ablation may prove to be a reasonable alternative to less nephron-sparing techniques. A series of four patients with transfusion-dependent tumor-related gross hematuria were treated successfully with RF ablation, with durable clinical effects.
MATERIALS AND METHODS
After written informed consent was obtained, all procedures were performed with a Radionics Cool-Tip 200-W RF ablation system (Radionics, Burlington, MA) under conscious sedation with midazolam and fentanyl. In three of four cases, RF ablation procedures were performed percutaneously under ultrasound (US) and computed tomography (CT) guidance, with the remaining procedure performed with US guidance alone with postprocedural CT. Relevant patient data are summarized in the Table. Age varied from 61 to 71 years. Baseline creatinine levels varied from 2.0 mg/dL to 3.7 mg/dL (normal values are 0.6-1.3 mg/dL for women and 0.8-1.5 mg/dL for men). All patients had received red blood cell transfusions in the days and hours before RF ablation.
Patient Data
| Creatinine (mg/dL) |
|||||||
|---|---|---|---|---|---|---|---|
| Pt. No./Sex/Age | Disease | PRBC Before RF Ablation | Before RF Ablation | After RF Ablation | Other Disease | Transcatheter Embolization | Previous Surgery |
| 1/M/64 | RCC with metastasis to liver and lung | 4 U over 3 days | 3.3 | 2.1 (8 months follow-up) | Hypertension, ARF, PCKD | Not considered due to poor renal function and the risk of contrast-induced nephropathy | None |
| 2/F/61 | RCC with metastasis to liver, lung, mediastinum | 12 U over 8 days | 1.7-3.7 | 1.1-1.4 (24 months follow-up) | None | Not considered due to poor renal function and the risk of contrast-induced nephropathy | Left nephrectomy for RCC |
| 3/M/71 | Metastatic melanoma to lung, kidney | 8 U over 10 days | 2.5 | 1.4 (9 months follow-up) | Prostate cancer | Not considered due to poor renal function and the risk of contrast-induced nephropathy | None |
| 4/M/71 | RCC with liver mets | >10 U over 6 weeks with continuous bladder irrigation | 2.0-2.5 | 1.0 (8 months follow-up) | CAD, CRF chemo-immunotherapy for lung metastases and a 7-cm right renal tumor | Unsuccessful selective embolization of two primary segmental feeders of a right kidney mass using PVA particles | Left nephrectomy for RCC |
ARF = acute renal failure; CAD = coronary artery disease; CRF = chronic renal failure; PCKD = polycystic kidney disease; PRBC = packed red blood cells; PVA = polyvinyl alcohol; RCC = renal cell carcinoma
Note.—All patients presented with gross hematuria.
In patient 1, a 17-gauge triple-cluster Radionics probe was placed into an upper-pole left renal tumor. Two sites within the upper pole were treated during two 12-minute sessions. Patient 2 had a 17-gauge triple-cluster Radionics probe placed into two 7-cm upper- and lower-pole right renal metastatic lesions for 22- and 18-minute sessions, respectively. Treatment times were longer than the standard 12 minutes because of low post-RF ablation tip temperatures, likely caused by high tumor tissue perfusion. Patient 3 presented with simultaneous extreme local pain in the right flank over the kidney before RF ablation, so RF ablation was chosen as a possible way to control tumor-related pain as well as hematuria. A 17.5-gauge triple-cluster Radionics probe was placed into three regions within the right kidney for a total of 33 minutes. For patient 4, complete embolization was not considered given a solitary kidney and underlying renal insufficiency. The patient refused surgical management. A 17.5-gauge, 3-cm active single-tip Radionics RF probe was placed into three slightly overlapping regions of the renal tumor for sessions of 12 minutes each.
RESULTS
Creatinine levels after RF ablation varied from 1.1 mg/dL to 2.1 mg/dL, but improved in all patients for a mean follow-up of 12 months (range, 8-24 months). No patient required transfusion for hematuria in the follow-up period. Two of four patients had an isolated transient episode of gross hematuria recurrence during follow-up, all of which resolved without repeat intervention.
Patient 1
Continuous bladder irrigation via catheter was performed for 24 hours after the procedure, with no blood clots seen in the urine after RF ablation. The patient had clear yellow urine at discharge from the hospital 1 day after RF ablation. Hematuria recurred 6 months after RF ablation, at which time urinalysis revealed gross hematuria (>40 red blood cells per high-power field).
However, the patient required only a single transfusion (1 U packed red blood cells) during 8 months of follow-up for chemotherapy-induced anemia. For the remainder of the last year of his life, he had microscopic hematuria and serum creatinine level was 2.1 mg/dL just before he died of cancer progression 8 months after RF ablation.
Patient 2
The patient tolerated the procedure well and did not experience significant pain. Discomfort was treated with oral analgesics as needed. Continuous bladder irrigation was performed for 48 hours after the procedure. No blood clots were seen in the urine after RF ablation. Gross hematuria resolved within 2 days after RF ablation and the patient had clear yellow urine at discharge from the hospital 5 days after RF ablation.
For the 24 months after RF ablation, urinalysis each month revealed microscopic hematuria (between 1-5 and 6-10 cells per high-power field). Macroscopic hematuria has not recurred to date, more than 24 months after RF ablation. Metastatic disease is stable, with stable creatinine levels ranging from 1.1 mg/dL to 1.4 mg/dL.
Patient 3
The day after the procedure, the patient had severe pain (average pain, 5 of 10 on a visual analog scale; worst pain, 7 of 10) and required a morphine patient-controlled analgesia pump immediately after the procedure. Four days after the procedure, the pain was under better control (1-2 of 10), the patient-controlled analgesia pump was discontinued, and pain was managed with oral morphine sulfate until discharge. Pain was reported as 0 of 10 one month after the procedure and as 1 of 10 three months after the procedure with no analgesic treatment 1-3 months after RF ablation. Continuous bladder irrigation was performed for 24 hours after the procedure, with resolution of gross hematuria and no evidence of blood clots. Urine analysis 4 days after RF ablation demonstrated 21-40 cells per high-power field.
The patient did not require additional transfusions after RF ablation. Ten days after RF ablation, the patient had a brief, self-limited episode of old clots or brown sloughed tissue in the urine resulting in bladder outlet obstruction, which was treated successfully with manual irrigation and clot irrigation. The patient died after 9 months, with the cause unrelated to the procedure.
Patient 4
Gross hematuria resolved during the 24 hours after RF ablation, and continuous bladder irrigation was discontinued 2 days thereafter. Gross hematuria resolved for 8 months, at which time it recurred and resolved without repeat intervention. Renal function remained stable during an 8-month follow-up period.
DISCUSSION
Forty percent of patients with renal malignancy can present with some degree of hematuria (3). Transfusion-dependent renal cancer-related hematuria may present a difficult clinical dilemma when renal preservation is desirable. Stopping hematuria may require parenchymal destruction with embolization or excision. These conventional options may exacerbate renal insufficiency. Nonsurgical candidates in whom conventional therapies fail have few options.
The incidence of renal cell carcinoma has recently shown a steady increase (4). Conventional management of renal neoplasms has consisted of complete nephrectomy. However, more recently, partial nephrectomy and laparoscopic nephrectomy have been shown to possibly offer a nephron-sparing option with similar efficacy (5). Patients with significant comorbidities, poor baseline renal function, and advanced metastatic disease are at higher risk with surgical management.
Transcatheter embolization has been described in the effective management of intractable gross hematuria related to bladder hemorrhage (6,7) and renal angiomyolipomas (1). Embolization can stabilize the patient’s condition acutely and provide long-term resolution of hematuria (1). Although selective embolization can preserve some renal function, it did not control hematuria in patient 4 in this series and may be technically difficult.
Less selective embolization can be more effective but may jeopardize renal function and normal parenchyma. Additionally, the inherent risk of contrast material-induced nephropathy may serve as a contraindication for patients who already have reduced renal function. Carbon dioxide and gadolinium have limitations as angiographic contrast agents.
RF ablation has been reported as a safe and effective method for focal destruction of small kidney tumors. RF ablation may also effectively cauterize tissue with minimal effects on renal function, and can result in durable resolution of clinically apparent hematuria (2).
Targeting the interface without completely destroying the tumor may still result in a durable response, perhaps because this destroys the communication between tumor and collecting system, although further validation studies are needed. Probes were placed in close proximity to the collecting system without actually entering the collecting system. In the case of the Radionics system, this means placing the needle tip into the tissue immediately adjacent to the collecting system, with the tip of the needle several millimeters from the junction of the collecting system and tumor.
Targeting the junction of tumor and collecting system with RF ablation (Figure) may result in an effective and durable response despite not ablating the entirety of the tumor. For example, in patient 4, a single-tip probe was chosen to treat a 7-cm tumor with three overlapping burns. Again, the goal was not to completely ablate the tumor, but rather to target the tumor-collecting system junction. Successful clinical outcome with relief of hematuria is possible without being overaggressive and subjecting patients to the risks inherent in attempting complete ablation of a 7-cm tumor. It is a risk/benefit ratio consideration.
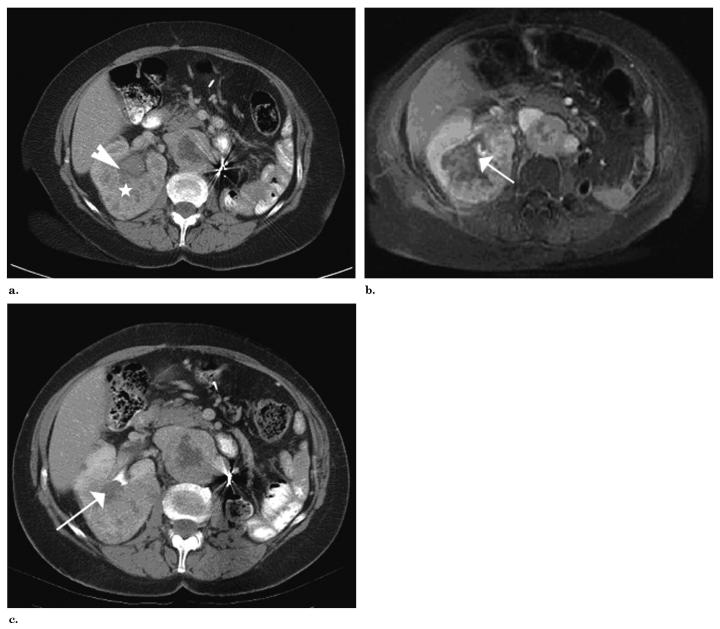
Figure.
(a) Enhanced CT of right kidney tumor after ablation details the proximity of the large tumor (white star) to the collecting system (white arrowhead). (b) T1-weighted, fat-saturated, gadolinium-enhanced MR after ablation shows treated area abutting collecting system (white arrow). (c) Contrast material-enhanced CT of right kidney 22 months after treatment demonstrates durable radiographic response, with interval shrinkage of treated region, but continued abutment of treated region and collecting system. The interface (white arrow) between the treated region and the collecting system remains devascularized.
Blood flow to the kidney is many times greater than that to the liver, and therefore there is likely more convective heat loss in the kidney than in the liver. This means that equal RF ablation parameters result in smaller burns in the kidney than in the liver. Therefore, more time and more overlapping of adjacent burns may be required in the kidney than in the liver. In the post-RF ablation period, aggressive bladder irrigation may be necessary to avoid bladder outlet obstruction from blood clots or sloughed tissue in the bladder.
Vigilance to possible signs of anuria or symptoms of hydronephrosis may be well-advised in the post-RF ablation period. Targeting the collecting system itself may increase the risk for urine leakage complications (eg, urinoma, fistula), although these are uncommon in renal tumor RF ablation.
The patients in this series had gross hematuria resulting in at least moderate anemia, transfusion dependence, and even profound hypotension in one case. All patients had comorbidities or advanced-stage neoplasms that precluded surgery. Additionally, these patients had poor renal function as a result of their primary disease and/or nephrectomy, making minimally invasive therapy more desirable.
For decades, RF devices have been used to achieve hemostasis in the surgical setting. Decreased blood loss during liver surgery has been described with RF ablation along surgical planes (8,9). RF ablation has also been described in the setting of hemobilia (10). A modified RF ablation device with a ball on the tip of the probe can be used for fulguration during surgery (Coleman J, Singh A, Pinto P, et al. Radiofrequency assisted laparoscopic partial nephrectomy in hereditary cancer: clinical and histologic results. September 2002-October 2003). In addition, modified RF ablation can be used on the outer needle during core biopsy to minimize bleeding after biopsy (11). The fact that RF ablation works for hematuria makes perfect sense: with coagulation necrosis, tissue does not bleed. Pre-RF ablation CT and magnetic resonance (MR) imaging may be important to attempt to localize the bleeding site to optimize RF ablation targeting in the case of large or multifocal tumors.
In this series, three patients were referred for RF ablation as primary management for gross transfusion-dependent hematuria. A fourth patient was referred for RF ablation as secondary management after failed selective embolization. In all four patients, RF ablation was effective in the acute management of gross hematuria related to neoplastic disease. Only one of the patients received a transfusion (1 U packed red blood cells) after RF ablation for unrelated anemia. No major complications were encountered, even though one patient presented 1 week after RF ablation with suprapubic distention and anuria. This was managed during a 1-day hospital stay with manual bladder irrigation, during which some moderate-sized blood clots were removed, likely a treatment effect from RF ablation. Transient gross hematuria recurred in two patients, which required no further intervention. Hospital stays varied between 1 and 5 days after RF ablation.
Pain after RF ablation was modest and was normally managed with oral medications as needed, even though one patient required a morphine patient-controlled analgesia pump for several days. However, this same patient had a dramatic resolution of his severe pre-RF ablation pain by 1 month after RF ablation and was able to discontinue analgesics. In all four patients, renal function measured by serum creatinine largely improved from pre-RF ablation values, manifesting the nephron-sparing nature of selective RF ablation.
RF ablation has specific limitations, including requiring the identification of a focal target, which is not requisite to successful embolization or resection. The potential risk for fistula or urinoma exists because the collecting system is in such close proximity to the target.
Although it is difficult to draw broad conclusions from a short retrospective series, RF ablation may be an effective therapeutic option for transfusion-dependent cancer-related hematuria in patients with renal insufficiency, solitary kidney, or comorbidities, or after failed conventional therapies in patients who are poor surgical candidates.
Abbreviation
- RF
radiofrequency
Footnotes
None of the authors have identified a conflict of interest.
References
- 1.Mourikis D, Chatziioannou A, Antoniou A, Kehagias D, Gikas D, Vlahos L. Selective arterial embolization in the management of symptomatic renal angiomyolipomas. Eur J Radiol. 1999;32:153–159. doi: 10.1016/s0720-048x(98)00179-x. [DOI] [PubMed] [Google Scholar]
- 2.Wood BJ, Grippo J, Pavlovich CP. Percutaneous radiofrequency ablation for hematuria. J Urol. 2001;166:2303–2304. [PMC free article] [PubMed] [Google Scholar]
- 3.Simons JW, Marshall FF. Kidney and ureter. In: Abeloff MD, Armitage JO, Lichter AS, Niederhuber JE, editors. Clinical oncology. second edition Churchill Livingstone; Philadelphia: 2000. pp. 1784–1799. [Google Scholar]
- 4.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 5.Ono Y, Kinukawa T, Hattori T, et al. Laparoscopic radical nephrectomy for renal cell carcinoma: a five-year experience. Urology. 1999;53:280. doi: 10.1016/s0090-4295(98)00505-6. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi T, Kusno S, Matsuayashi T, Uchida T. Selective embolization of the vesical artery in the management of massive bladder hemorrhage. Radiology. 1980;136:345–348. doi: 10.1148/radiology.136.2.7403507. [DOI] [PubMed] [Google Scholar]
- 7.Terrence D, Schuhrke, John W. Barr Intractable bladder hemorrhage: therapeutic angiographic embolization of the hypogastric arteries. J Urol. 1976;116:523–525. doi: 10.1016/s0022-5347(17)58892-8. [DOI] [PubMed] [Google Scholar]
- 8.Weber JC, Navarra G, Jiao LR, Nicholls JP, Jensen SL, Habib NA. New technique for liver resection using heat coagulative necrosis. Ann Surg. 2002;236:560–563. doi: 10.1097/00000658-200211000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchizaki U, Miyamori H, Kitagawa S, Kaneko S. Radiofrequency ablation for life-threatening ruptured hepatocellular carcinoma. J Hepatol. 2004;40:354–355. doi: 10.1016/j.jhep.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Kim RY, Weintraub JL, Susman J, Haskal ZJ. Radiofrequency ablation for hemobilia secondary to hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13:317–320. doi: 10.1016/s1051-0443(07)61726-6. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard WF, Wray-Cahen D, Karanian JW, Hilbert S, Wood BJ. Radiofrequency cauterization with biopsy introducer needle. J Vasc Interv Radiol. 2004;15:183–187. doi: 10.1097/01.rvi.000019398.74740.69. [DOI] [PMC free article] [PubMed] [Google Scholar]