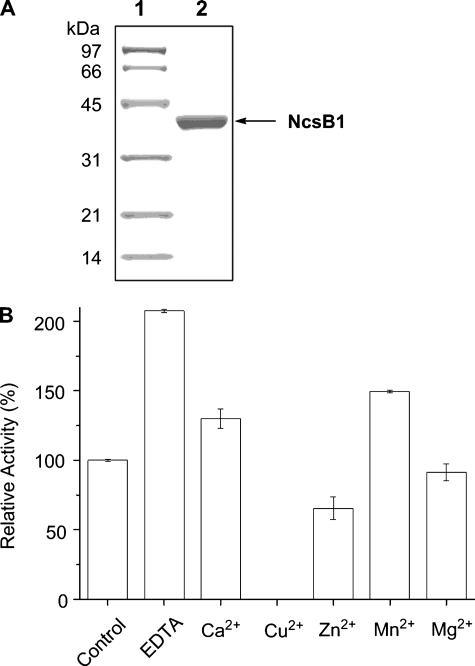
FIGURE 2.
NcsB1 and optimization of its O-methyltransferase activity in vitro. A, NcsB1 purified from E. coli BL21(DE3)/pBS5039 used in this study as judged by analysis of a 12% SDS-PAGE (lane 1, low range molecular weight protein standards; lane 2, purified His6-tagged NcsB1 with the predicted molecular mass of 39.5 kDa); and B, effect of 5 mm divalent metals on NcsB1-catalyzed O-methylation of 2,7-dihydroxy-5-methyl-1-naphthoic acid (8) in vitro.