Abstract
A conformationally altered prelatent form of antithrombin that possesses both anticoagulant and antiangiogenic activities is produced during the conversion of native to latent antithrombin (Larsson, H., Akerud, P., Nordling, K., Raub-Segall, E., Claesson-Welsh, L., and Björk, I. (2001) J. Biol. Chem. 276, 11996–12002). Here, we show that the previously characterized prelatent antithrombin is a mixture of native antithrombin and a modified, true prelatent antithrombin that are resolvable by heparin-agarose chromatography. Kinetic analyses revealed that prelatent antithrombin is an intermediate in the conversion of native to latent antithrombin whose formation is favored by stabilizing anions of the Hofmeister series. Purified prelatent antithrombin had reduced anticoagulant function compared with native antithrombin, due to a reduced heparin affinity and consequent impaired ability of heparin to either bridge prelatent antithrombin and coagulation proteases in a ternary complex or to induce full conformational activation of the serpin. Significantly, prelatent antithrombin possessed an antiangiogenic activity more potent than that of latent antithrombin, based on the relative abilities of the two forms to inhibit endothelial cell growth. The prelatent form was conformationally altered from native antithrombin as judged from an attenuation of tryptophan fluorescence changes following heparin activation and a reduced thermal stability. The alterations are consistent with the limited structural changes involving strand 1C observed in a prelatent form of plasminogen activator inhibitor-1 (Dupont, D. M., Blouse, G. E., Hansen, M., Mathiasen, L., Kjelgaard, S., Jensen, J. K., Christensen, A., Gils, A., Declerck, P. J., Andreasen, P. A., and Wind, T. (2006) J. Biol. Chem. 281, 36071–36081), since the 1H NMR spectrum, electrophoretic mobility, and proteolytic susceptibility of prelatent antithrombin most resemble those of native rather than those of latent antithrombin. Together, these results demonstrate that limited conformational alterations of antithrombin that modestly reduce anticoagulant activity are sufficient to generate antiangiogenic activity.
Antithrombin and its glycosaminoglycan activators, heparin and heparan sulfate, are well established anticoagulant regulators of blood clotting proteases (1–3). Antithrombin acts as an anticoagulant by irreversibly inhibiting clotting proteases through a conformational trapping mechanism that is unique to the serpin superfamily of proteins of which antithrombin is a member (4, 5). Heparin and heparan sulfate are required to activate antithrombin to ensure that clotting proteases are inhibited at a physiologically significant rate. This activating effect is the basis for the widespread clinical use of heparin for anticoagulant therapy. The activation results from heparin binding to antithrombin through a specific pentasaccharide sequence and inducing a conformational change in the serpin (6, 7). Conformational activation greatly enhances the affinity of antithrombin for heparin and exposes exosites on the inhibitor that promote its interaction with the target proteases, factor Xa and factor IXa (8–11). Heparin additionally accelerates antithrombin-protease reactions by providing a bridging exosite for the protease to bind next to antithrombin and thereby promote its interaction with the serpin in a ternary complex with heparin (12–14). The latter is the predominant mechanism involved in accelerating antithrombin inhibition of thrombin.
Antithrombin has more recently been shown to express a potent antiangiogenic activity after having undergone conformational alterations induced either by limited proteolysis in a reactive protease binding loop or by mild heating (15, 16). Such conformational alterations transform the native metastable protein to a much more stable but inactive form in which the reactive loop has inserted into the major β-sheet, the A-sheet, of the serpin (17, 18). These conformationally altered forms of antithrombin are produced under physiologic conditions (19) and have antiangiogenic activity comparable with that of other naturally produced angiogenesis inhibitors (20). The requirement for conformational change to generate antiangiogenic activity sets antithrombin apart from other serpins, such as pigment epithelium-derived factor, maspin, and kallistatin, which have been shown to also possess antiangiogenic activity but without the need for conformational change (21–23). Interestingly, mild heat treatment was also found to produce a distinct form of antithrombin, termed the prelatent form, which possessed antiangiogenic activity (24). However, unlike the cleaved and latent forms of antithrombin that have lost their ability to inhibit clotting proteases, prelatent antithrombin was found to retain clotting protease inhibitory activity and to have its reaction with these proteases accelerated by heparin. The only reported difference between the native and prelatent forms of antithrombin was a greater susceptibility of the latter to proteolysis by nontarget proteases. Since these findings suggested that an antiangiogenic epitope may be generated by more limited conformational changes than those having occurred in the cleaved and latent forms of antithrombin, it has been of interest to characterize the nature of these conformational differences between then native and prelatent forms of the serpin.
In the present report, we show that prelatent antithrombin generated as in past studies is actually a mixture of a novel antiangiogenically active species of antithrombin, the true prelatent form, and the antiangiogenically inactive native serpin. The purified prelatent antithrombin has a more potent antiangiogenic activity than latent or cleaved antithrombins but retains the anticoagulant functions of the native serpin. It is shown to be generated as an intermediate on the pathway to latent antithrombin in the presence of stabilizing anions of the Hofmeister series (25, 26). Significantly, only limited conformational alterations are involved in transforming native to prelatent antithrombin, as judged from the modest changes in heparin affinity, heparin-induced conformational activation, thermal stability, electrophoretic mobility, proteolytic susceptibility, and 1H NMR spectrum. Overall, our findings suggest that limited conformational changes, comparable with those recently demonstrated in a prelatent form of the serpin, plasminogen activator inhibitor-1 (27), are required to express antiangiogenic activity in antithrombin.
EXPERIMENTAL PROCEDURES
Proteins—Human α-antithrombin was purified from blood plasma, as previously described (28, 29). The concentration of the protein was determined from the 280 nm absorbance based on a molar absorption of 37,700 m-1 cm-1 (30). Reactive loop-cleaved antithrombin was prepared by incubating native antithrombin with human neutrophil elastase (Athens Research Technology) as in previous studies (31, 32). Human thrombin was prepared from plasma-purified prothrombin (33) by activating the zymogen and purifying the active protease as described (34). Human factor Xa was purchased from Enzyme Research (South Bend, IN). Concentrations of proteases were assessed from their activities in standard assays with peptidyl-p-nitroanilide chromogenic substrates and were based on calibration of these assays with active site-titrated enzymes (10).
Heparin—Full-length heparin chains of ∼26 saccharides or ∼50 saccharides with reduced polydispersity and with high affinity or low affinity for antithrombin were isolated from commercial heparin, as described (35). The synthetic pentasaccharide corresponding to the antithrombin binding sequence in high affinity heparin chains was generously provided by Maurice Petitou (Sanofi-Aventis, Toulouse, France). Concentrations of high affinity heparins were determined from stoichiometric titrations of antithrombin with the heparins as described previously (28). Low affinity heparin concentrations were measured by Azure A dye binding assays (35).
Preparation of Prelatent and Latent Forms of Antithrombin— Two slightly different procedures were used for isolating prelatent antithrombin. In the first, antithrombin (∼10 mg at 3 mg/ml) in 10 mm Tris-HCl, 0.5 m sodium citrate, pH 7.4, was incubated at 60 °C for 24 h as in previous studies (24). The protein was then dialyzed against 20 mm sodium phosphate, 0.02 m NaCl, 0.1 mm EDTA, pH 7.4, buffer, and the dialyzed protein was applied to a 5-ml Hi-Trap heparin column at 0.5 ml/min. The column was subsequently washed with buffer with no NaCl at 0.5–2 ml/min until the protein fluorescence, detected continuously with a Shimadzu fluorescence monitor (280-nm excitation and 340-nm emission wavelengths), reached base-line level. A linear salt gradient from 0 to 2 m NaCl in sodium phosphate buffer was then applied over 45 min at 1 ml/min to elute the antithrombin. The low salt-eluting peak (∼0.2 m) comprising latent antithrombin was pooled, concentrated, and dialyzed. The high salt-eluting peak, previously designated as prelatent antithrombin, was subdivided into leading and trailing edge pools (labeled A + B and C, respectively) at the center of the peak (see Fig. 1). The leading edge pool was concentrated by ultrafiltration, dialyzed into sodium phosphate buffer, pH 7.4, containing 20 mm NaCl, and rechromatographed as above except that the protein was eluted with a 0–2 m convex NaCl gradient over 50 min at 1 ml/min. The two partly resolved peaks that eluted were separately pooled by subdividing at the trough between the two peaks and designated pools A and B in order of their elution.
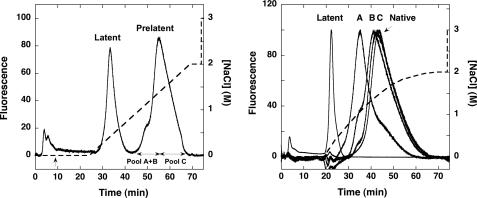
FIGURE 1.
Isolation of prelatent antithrombin by heparin-agarose chromatography. Left, elution profile of heat-treated native plasma antithrombin after chromatography on a Hi-Trap heparin column (solid line). Protein was eluted from the column with a linear NaCl gradient (dashed line) and was detected by intrinsic fluorescence. The protein peak eluting between 1 and 2 m NaCl was subdivided as shown into two pools, and the leading edge pool was rechromatographed and similarly subdivided to obtain the three pools A, B, and C (A–C), as described under “Experimental Procedures.” Right, elution profiles of antithrombin pools A, B, and C after rechromatography on the Hi-Trap Heparin column using a convex salt gradient (dashed line). Latent antithrombin and untreated native antithrombin were similarly chromatographed. All chromatograms were corrected for background fluorescence by subtracting a chromatogram of buffer alone. Further details are provided under “Experimental Procedures.”
In the second procedure, later adopted for optimizing the chromatographic separation and yield of prelatent antithrombin, the preparation was done as above except that antithrombin was incubated at 60 °C for 30 h instead of 24 h prior to dialysis and Hi-Trap heparin chromatography. Moreover, after washing of the column, the NaCl concentration was increased to 0.1 m, and the flow rate was increased to 2.5 ml/min to elute latent antithrombin and allow the fluorescence to return to base line. The prelatent antithrombin was then eluted using the following linear gradient program at 2.5 ml/min: 0.1 m NaCl wash for 0–5 min, 0.1–0.88 m NaCl gradient from 5 to 30 min, hold at 0.88 m NaCl for 10 min, and 0.88–3 m NaCl gradient from 40 to 65 min. The shoulder on the high salt-eluting peak was pooled, concentrated, and dialyzed into low salt buffer and then rechromatographed using the same program. In some cases, two separate samples were successively processed as above, and the shoulders from both runs were combined prior to the second chromatography step. The peak eluting prior to the major protein peak was pooled to obtain prelatent antithrombin.
Kinetics of Prelatent Antithrombin Formation—The kinetics of formation of prelatent and latent antithrombins from native antithrombin were assessed by heating the native serpin in the Tris/citrate buffer at 60 °C as above for varying times and then chromatographing samples on the Hi-Trap heparin column using the convex salt gradient. Corrections for background fluorescence were made by subtracting a buffer blank run from all chromatograms. The fluorescence peaks corresponding to native, prelatent, and latent antithrombins were then integrated using Millenium software (Waters Corp.) to quantitate their relative amounts. Minor fluorescence peaks corresponding to protein that was not bound or weakly bound to the column were integrated and summed to account for a nonbinding fraction. To determine whether prelatent antithrombin was an intermediate in the formation of latent antithrombin, purified prelatent antithrombin (0.16 mg/ml) was dialyzed into the Tris/citrate buffer and incubated under the same conditions used to form the prelatent species. Samples were taken at varying times, dialyzed, and chromatographed on the Hi-Trap heparin column as in the studies with native antithrombin. The kinetic stability of purified latent antithrombin was analyzed similarly. Reaction progress curves were fit by the minimal reaction model of Fig. 4 by numerical integration of the differential equations for the model using Scientist software (Micromath, Inc.).
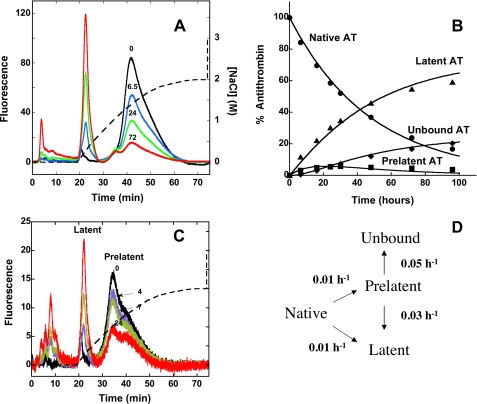
FIGURE 4.
Kinetics of conversion of native antithrombin to prelatent and latent forms. A, fluorescence elution profiles obtained after heating native plasma antithrombin at 60 °C in pH 7.4 citrate buffer for the indicated times (in h) and chromatographing 10 μg samples on the Hi-Trap heparin column as in Fig. 1 using the convex salt gradient (dashed line). The different forms of antithrombin eluting from the column were quantitated by integrating the areas under each peak, and the relative amounts of each form were expressed as a percentage of the total fluorescence. Further details are provided under “Experimental Procedures.” B, plot of the time dependence for conversion of native antithrombin (AT) (•) to prelatent (▪), latent (▴), and nonbinding (♦) forms based on the quantitation of these forms in the chromatograms of A and others not shown for clarity. The solid lines indicate the computer fit of data by the model in D along with the fitted rate constants as detailed under “Experimental Procedures.” C, chromatograms of isolated prelatent antithrombin after incubating at 60 °C in pH 7.4 Tris/citrate buffer for the indicated times (in h) and chromatographing on Hi-Trap Heparin with elution using the convex salt gradient (dashed line). Prelatent, latent, and unbound forms of antithrombin were quantitated by integration of peaks and normalizing to the total fluorescence as in A. D, minimal kinetic model consistent with the data of A–C together with the rate constants providing the best fit of the data by this model.
Alternative conditions that were tested for producing prelatent antithrombin involved (i) incubating 3 mg/ml antithrombin samples in 10 mm MES,2 pH 6, 10 mm Tris, pH 7.4, or 10 mm TAPS, pH 9, buffers, each containing 0.5 or 1 m sodium citrate, at60 °C for 30 h at pH 6 and 7.4 or 7 h at pH 9; (ii) incubating 0.5 mg/ml antithrombin samples in 50 mm Tris, 50 mm NaCl, pH 7.5, buffer containing 20% glycerol at 60 °C for 24–48 h (19); (iii) incubating 0.2 mg/ml antithrombin samples at 25 °C for 24 h in Tris/sodium citrate buffer containing 0, 0.5, 1, 3, or 6 m guanidine HCl followed by dialysis into ionic strength (I) 0.15 sodium phosphate buffer, pH 7.4 (36); (iv) incubating 1–3 mg/ml antithrombin samples in 10 mm Tris, pH 7.4, containing 0.1, 0.25, 0.5, or 1 m citrate, 0.5 m sodium phosphate, 0.5 m EDTA, 3 m NaCl, or 100 μm heparin pentasaccharide at 60 °C for 30 h (26); and (v) incubating 2 μm antithrombin samples in a physiologic buffer consisting of 9.47 mm sodium phosphate, 137 mm NaCl, 2.5 mm KCl, 1 mm CaCl2, pH 7.4 (37), in the absence or presence of 1 μm 50-saccharide high affinity heparin or 15 μg/ml heparan sulfate (bovine kidney; Sigma) at 60 °C for a time yielding ∼50% inactivation of antithrombin. Samples were diluted 50–100-fold into sodium phosphate buffer with no salt before chromatography of 20–50 μg as above except for samples containing heparin. The latter were first chromatographed on the monoQ column to remove heparin (29) and then, after concentration and dilution, chromatographed on the Hi-Trap heparin column.
Experimental Conditions—Experiments were conducted at 25 °C in I 0.15, pH 7.4 buffers consisting of either (i) 20 mm sodium phosphate, 0.1 m NaCl, 0.1 mm EDTA, 0.1% polyethylene glycol 8000 or (ii) 0.1 m Hepes, 0.1 m NaCl, 5 mm CaCl2, 0.1% polyethylene glycol 8000, except where otherwise noted. Some experiments were performed in the sodium phosphate buffer but with no added NaCl or with the addition of 0.25 m NaCl to yield ionic strengths of 0.05 or 0.3, respectively.
PAGE—Native PAGE and SDS-PAGE analysis of proteins was done as in past studies using the Laemmli discontinuous buffer system (38). The susceptibility of different antithrombin forms to proteolysis by nontarget proteases was analyzed essentially according to previous work (24) by incubation of 200 μg/ml serpin with 20 μg/ml chymotrypsin or thermolysin in 10 mm Tris, 5 mm CaCl2, pH 7.4, buffer for varying times, followed by quenching with 1.5 mm phenylmethylsulfonyl fluoride for chymotrypsin reactions or 30 mm EDTA for thermolysin reactions. Samples were denatured by the addition of SDS treatment buffer and boiling and were then analyzed for proteolysis by SDS-PAGE.
Affinity and Kinetics of Antithrombin-Heparin Interactions— Binding of pentasaccharide or ∼26-saccharide high affinity heparins to antithrombin was analyzed by fluorescence titrations in which the enhancement in protein tryptophan fluorescence accompanying polysaccharide binding was used to monitor the interaction (28). Titrations were done with 50–200 nm antithrombin in I 0.15 or I 0.3 sodium phosphate buffers for measurements of KD or in I 0.05 sodium phosphate buffer for measurements of binding stoichiometry. Measurements were made on a SLM 8000 spectrofluorometer, at an excitation wavelength of 280 nm and an emission wavelength of 340 nm. Titrations were analyzed by computer fitting to the quadratic binding equation with KD, the maximal fluorescence change, and the stoichiometry as the fitted parameters (28). For measurements of KD, the stoichiometry was fixed at the average fitted value obtained in titrations at low ionic strength.
The kinetics of heparin pentasaccharide binding to antithrombin were analyzed under pseudo-first order conditions with an Applied Photophysics SX-17MV stopped-flow fluorometer as in past studies (7, 29). Variable concentrations of pentasaccharide were mixed with antithrombin at a concentration at least 5-fold lower than that of the saccharide in I 0.3 buffer. Pentasaccharide binding to the protein was monitored from increases in protein fluorescence, and the observed pseudo-first order rate constant (kobs) was determined by computer fitting of the progress curves to a single exponential function.
Stoichiometries of Protease Inhibition—Fixed concentrations of protease (50 nm) were incubated with variable concentrations of antithrombin ranging from substoichiometric to approximately equimolar in I 0.15, pH 7.4, sodium phosphate buffer. After incubation for a time sufficient to yield >90% inactivation based on measured inhibition rate constants, residual enzyme activities were determined as in the kinetic assays described below. Plots of residual enzyme activity versus the molar ratio of inhibitor to enzyme were fit by linear regression to obtain the inhibition stoichiometry from the abscissa intercept.
Kinetics of Protease Inhibition—The kinetics of high affinity heparin and low affinity heparin acceleration of antithrombin-protease reactions were measured under pseudo-first order conditions as in past studies (35). Antithrombin (20 or 50 nm) was reacted at 25 °C with protease at one-tenth (factor Xa reactions) or one-twentieth (thrombin reactions) the concentration of inhibitor in the presence of variable concentrations of heparin. The buffers employed were either I 0.15 sodium phosphate (thrombin reactions) or I 0.15 Hepes/CaCl2 (factor Xa reactions), pH 7.4. Reactions with thrombin and high affinity heparin additionally contained 2 mm p-aminobenzamidine to decrease the rate of the reaction so as to allow accurate kinetic measurements by the discontinuous sampling method. Reaction mixtures (50–100 μl) were incubated for fixed times (2-min reactions with thrombin and high affinity heparin, 5-min reactions with thrombin and low affinity heparin, 30-s reactions with factor Xa and high affinity heparin and 5-min reactions with factor Xa and low affinity heparin) or for variable times to obtain full progress curves (factor Xa reactions with low affinity heparin) in polyethylene glycol-coated polystyrene cuvettes. The reactions were then quenched with substrate (900–950 μl), either 50 μm tosyl-Gly-Pro-Arg-7-amido-4-methylcoumarin (Sigma) for thrombin reactions or 100 μm Spectrozyme FXa (American Diagnostica) for factor Xa reactions. The substrate was in a high salt buffer consisting of 20 mm sodium phosphate, 0.1 mm EDTA or 100 mm Hepes, both at pH 7.4, and with 1 m NaCl, 0.1% polyethylene glycol 8000, 0.1 mg/ml Polybrene, to ensure quenching of the heparin-catalyzed reaction. The residual enzyme activity was determined by monitoring for several minutes the initial linear rate of substrate hydrolysis either from the increase in 7-amido-4-methylcoumarin fluorescence at λex 380 nm, λem 440 nm (for thrombin reactions) or the increase in p-nitroaniline absorbance at 405 nm (for factor Xa reactions). Observed pseudo-first order rate constants (kobs) were obtained from the fractional decrease of the enzyme activity from the starting activity measured in the absence of inhibitor by assuming an exponential loss of activity with zero end point (28). Apparent second order rate constants (kapp) were then calculated from kobs by dividing by the functional inhibitor concentration. The latter was determined by multiplying the inhibitor concentration measured from the 280 nm absorbance by the reciprocal of the inhibition stoichiometry (25). The functional inhibitor concentration thus obtained agreed with the 0.8–0.9-mol fraction of antithrombin found to be active in binding heparin from stoichiometric heparin binding titrations (Table 2). The dependence of kapp on heparin concentration was fit by the ternary complex bridging model for all reactions (35) except for the low affinity heparin-catalyzed reaction of antithrombin with factor Xa. The latter was fit by a rectangular hyperbolic function, based on a model in which heparin catalysis results solely from conformational activation of antithrombin with no contribution of bridging (35). This model is suggested from the observation that low affinity heparin rate enhancements were indistinguishable in buffers expected to promote bridging (Hepes/CaCl2) or in which bridging was minimal (sodium phosphate) (39).
TABLE 2.
Binding of pentasaccharide (H5) and full-length (H26) high affinity heparins to native and prelatent antithrombins Native and prelatent antithrombins were titrated with the indicated high affinity heparins under stoichiometric binding conditions (I 0.05) or under conditions where KD was well determined (I 0.15 or I 0.3) as indicated below and in Fig. 5. Binding was monitored from increases in tryptophan fluorescence as in previous studies, and binding parameters were determined by fitting titration curves by the equilibrium binding equation, as described under “Experimental Procedures.” Binding stoichiometries determined in I 0.05 buffer were fixed in fits of titrations in I 0.15 or I 0.3 buffers. Only an upper limit for KD in I 0.05 buffer is provided, because binding was too tight to measure accurately in these titrations. Errors represent ± S.E. for 3–7 titrations.
| Antithrombin | Heparin | I | Stoichiometry | KD | ΔFmax/Fo |
|---|---|---|---|---|---|
| mol AT/mol H | nm | ||||
| Native | H5 | 0.05 | 0.89 ± 0.03 | <10 | 0.41 ± 0.01 |
| Prelatent | H5 | 0.05 | 0.79 ± 0.05 | <10 | 0.35 ± 0.01 |
| Native | H5 | 0.15 | 34 ± 3 | 0.39 ± 0.01 | |
| Prelatent | H5 | 0.15 | 58 ± 3 | 0.34 ± 0.01 | |
| Native | H26 | 0.15 | 20 ± 3 | 0.42 ± 0.02 | |
| Prelatent | H26 | 0.15 | 39 ± 4 | 0.34 ± 0.02 | |
| Native | H5 | 0.30 | 480 ± 23 | 0.39 ± 0.01 | |
| Prelatent | H5 | 0.30 | 880 ± 84 | 0.34 ± 0.01 |
Uncatalyzed rates of protease inhibition by antithrombin in the absence of heparin were similarly analyzed under pseudo-first order conditions in the same buffers but with the addition of 0.1 mg/ml Polybrene to neutralize any trace heparin in the incubation. Reactions of 100 nm antithrombin with 5 nm protease were quenched at varying times by 10-fold dilution into reaction buffer containing 100 μm Spectrozyme FXa (factor Xa reactions) or 100 μm S-2238 (thrombin reactions). The residual enzyme activity was determined by measuring the initial rate of substrate hydrolysis at 405 nm. The time-dependent decrease in enzymatic activity was fit by an exponential function with a zero activity end point to obtain kobs.
Heparin pentasaccharide-catalyzed reactions of 50–200 nm antithrombin with 3 nm factor Xa were similarly done as a function of pentasaccharide concentration (0–10 nm) for fixed reaction times of 5 or 10 min. The loss of enzyme activity was fit by an exponential function with zero end point with heparin concentration as the independent variable to obtain the second order rate constant for the reaction of antithrombin-pentasaccharide complex with factor Xa as described (9).
Melting Temperature Determination—Thermal denaturation analysis of antithrombins was performed over the temperature range 40–75 °C. Measurements were made on an SLM 8000 spectrofluorometer at λex 280 nm and λem 340 nm and with the temperature regulated in a Peltier-controlled cuvette holder (Quantum Northwest, Spokane, WA). Melting curves were analyzed by nonlinear regression fitting to the van't Hoff equation (40, 41) to generate the melting temperature (Tm) for native and prelatent antithrombins.
NMR Studies—Native, prelatent, or latent antithrombin samples were exchanged into I 0.15 sodium phosphate buffer containing 99.9% D2O by repeated dilution and concentration by ultrafiltration. 1H spectra of equal concentrations of protein (∼10 μm) were acquired on a 900-MHz Bruker instrument with cryoprobe for ∼1 h at 25 °C.
Cell Culture—Human umbilical vein endothelial cells (HUVECs) were purchased from Cascade Biologics (Portland, OR) and maintained in 2% low serum growth supplement (Cascade Biologics) as described in the manufacturer's protocol at 37 °C in an atmosphere of 5% CO2, 95% air.
Cell Proliferation Assay—HUVECs were seeded in 96-well plates at a density of 4000 cells/well in reduced fetal bovine serum (0.2%). After 18 h of starving, the cells were exposed for 48 h to 10 ng/ml FGF-2 (Invitrogen) in the presence or absence of antithrombins. Cells were then incubated with 20 μl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution (Promega, Madison, WI) for 1–4 h at 37 °C, and the absorbance at 490 nm was measured.
RESULTS
Isolation of Prelatent Antithrombin—Mild heating of native antithrombin at 60 °C for 24 h in the presence of the stabilizing agent, citrate (17, 26), followed by chromatography on heparin-agarose resulted in two major antithrombin peaks (Fig. 1, left), as previously reported (24). One peak, eluting at low salt concentration, corresponded to the anticoagulantly inactive, but antiangiogenically active, latent form of the serpin. The other peak, eluting at high salt concentration, corresponded to what was previously termed “prelatent” antithrombin, because it was shown to be both anticoagulantly and antiangiogenically active and therefore was thought to be an intermediate on the pathway to latent antithrombin. With continuous fluorescence monitoring of the protein that eluted from the column, we reproducibly observed a distinct shoulder at the leading edge of the prelatent peak eluting at high salt, suggesting that this peak consisted of at least two distinct forms of antithrombin (42). To verify this, the prelatent antithrombin peak was subdivided into leading and trailing edge pools (the latter designated pool C). The leading edge pool was then rechromatographed under the same conditions, and the eluted protein was again subdivided into leading edge (pool A) and trailing edge (pool B) pools, to produce three separate pools. Rechromatography of each of the three pools using a convex salt gradient to improve resolution showed that a distinct lower heparin affinity species was enriched in the pools derived from the leading edge pool of the original prelatent peak (Fig. 1, right). A higher heparin affinity species indistinguishable in its elution position from untreated plasma antithrombin was correspondingly reduced in the same pools.
Native PAGE analysis showed that all three pools migrated similarly to native antithrombin and with a lower mobility than that of latent antithrombin, indicating that prelatent and native antithrombin forms were highly similar in their conformation and overall charge (Fig. 2). The protease-inhibitory function was tested by assessing the ability of the different pools to form complexes with thrombin by SDS-PAGE. All antithrombin pools behaved like native antithrombin in showing complete shifts of the antithrombin band to a higher molecular weight band, after exposure to a molar excess of thrombin, corresponding to a 1:1 antithrombin-thrombin covalent complex (Fig. 2). The latent antithrombin was, as expected, unable to form a complex with thrombin, consistent with complete loss of its protease inhibitory activity. Kinetic assays of antithrombin inhibition of thrombin enzymatic activity under pseudo-first order conditions indicated similar second order association rate constants for antithrombin pools A–C (Table 1). End point titrations of thrombin with antithrombin also produced similar inhibition stoichiometries for the three antithrombin pools (Table 1), consistent with the SDS-PAGE analysis of inhibition.
FIGURE 2.
SDS and native PAGE characterization of prelatent antithrombin. Shown are the electrophoretic gels of antithrombin pools A, B, and C (5 μg of protein) obtained during the purification of prelatent antithrombin by Hi-Trap heparin chromatography (Fig. 1) under denaturing (SDS) and native conditions. Native and latent antithrombin samples were run as controls. The ability of each antithrombin (AT) pool to form an SDS-stable complex with a molar excess of thrombin (5 μg) is shown in the SDS gel.
TABLE 1.
Stoichiometries and kinetics of inhibition of thrombin and factor Xa by native and prelatent antithrombins Untreated antithrombin (AT) and the different pools obtained from Hi-Trap heparin chromatography of heat-treated antithrombin were compared with respect to (i) the stoichiometries of inhibition (SI) of thrombin (IIa) and (ii) the second order rate constants for the inhibition of thrombin or factor Xa (FXa) in the absence of heparin (kuncat) or for the inhibition of factor Xa in the presence of saturating heparin pentasaccharide (kH5) as described under “Experimental Procedures.” Errors represent S.E. obtained from the fits of stoichiometric titrations or reaction kinetic curves as a function of time or heparin concentration.
| Antithrombin | SI (IIa) | kuncat (IIa) | kuncat (FXa) | kH5 (FXa) |
|---|---|---|---|---|
| mol AT/mol IIa | m–1 s–1 | m–1 s–1 | m–1 s–1 | |
| Native AT | 1.20 ± 0.04 | 7.2 ± 0.3 × 103 | 2.1 ± 0.1 × 103 | 5.6 ± 0.2 × 105 |
| Pool A | 1.22 ± 0.14 | 7.0 ± 0.2 × 103 | 1.9 ± 0.1 × 103 | 5.1 ± 0.1 × 105 |
| Pool B | 1.15 ± 0.14 | 7.7 ± 0.2 × 103 | 5.1 ± 0.3 × 105 | |
| Pool C | 1.02 ± 0.08 | 7.4 ± 0.2 × 103 | 5.4 ± 0.3 × 105 |
The antiangiogenic activity of the highest heparin affinity pool C and lowest heparin affinity pool A, the pools that were most enriched in a single species, was tested by measuring the ability to inhibit FGF-2-stimulated endothelial cell proliferation. A significant antiproliferative activity was found in pool A, whereas no activity was detected in pool C (Fig. 3). These findings suggest that the originally reported prelatent antithrombin fraction was actually a mixture of untransformed native antithrombin that lacked antiangiogenic activity and a somewhat lower heparin affinity species that possessed antiangiogenic activity.
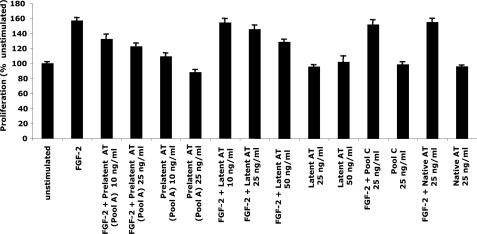
FIGURE 3.
Antiproliferative activity of prelatent antithrombin. HUVECs were cultured with or without stimulation by FGF-2 and in the absence or presence of prelatent (pool A), latent, and native (untreated and pool C) forms of antithrombin (AT) (see Fig. 1) as indicated for 48 h. The number of viable cells was then assayed colorimetrically. Triplicate assays were performed for each condition, and results were expressed relative to the unstimulated control. Further details are provided under “Experimental Procedures.” Error bars, S.E. values obtained after grouping results from several independent experiments. Prelatent and latent antithrombins produced statistically significant growth inhibition (p < 0.01) at minimal doses of 10 and 50 μg/ml, respectively.
Subsequent preparations of the prelatent species employed modifications in the gradient program for elution from heparin-agarose to further optimize its resolution (see “Experimental Procedures”). The amount of residual native antithrombin in the purified prelatent antithrombin was estimated by rechromatographing the protein, subdividing the peak at the center into leading and trailing edge pools, and then rechromatographing each subpool once more on the heparin affinity matrix. The two subpools eluted with indistinguishable peak positions and differed only slightly in their trailing ends (not shown). Based on the difference between the two elution peaks, native antithrombin was estimated to comprise less than 10% of the prelatent antithrombin.
Kinetics of Formation of Prelatent Antithrombin—To determine whether the antiangiogenically active prelatent antithrombin species was an intermediate in the conversion of native to latent antithrombin, we investigated the kinetics of its formation. Native antithrombin was heat-treated for varying times at pH 7.4 and chromatographed on heparin-Sepharose, and the rate of transformation of native antithrombin to prelatent and latent forms was assessed by integrating the fluorescence peaks corresponding to these species. The disappearance of native antithrombin was accompanied by the appearance of a dominant latent peak and the initial appearance and subsequent disappearance of a less prominent prelatent peak over ∼100 h (Fig. 4A). Small amounts of antithrombin, which bound weakly or not at all to the column, also accumulated over the reaction time course. The disappearance of native antithrombin followed a single exponential decay (t½ ∼ 30 h) with ∼75% of the reacted inhibitor concomitantly appearing as the latent species (Fig. 4B). Significantly, there was no lag in the appearance of latent antithrombin, as would be expected if prelatent antithrombin was an obligate intermediate in its formation.
To determine whether the prelatent species could convert to the latent form, purified prelatent antithrombin was dialyzed into the pH 7.4 Tris/citrate buffer and heated at 60 °C as in the experiments with native antithrombin. Prelatent antithrombin was clearly transformed to the latent species as well as species that bound weakly or not at all to the column (Fig. 4C). In contrast, a similar dialysis and heat treatment of purified latent antithrombin revealed that the latent species was extremely stable once formed and did not convert to the low heparin affinity species formed in the reactions of native or prelatent antithrombins (not shown). These observations suggested that native antithrombin converts either directly to the latent species or indirectly to the latent form through a prelatent antithrombin intermediate. The prelatent species in turn transforms either to the latent form or forms that bind weakly to the heparin affinity matrix (Fig. 4D). The latter were monomeric forms that appeared indistinguishable from latent antithrombin on native PAGE, but which eluted from the Hi-Trap heparin column in the starting buffer even after concentration, dialysis, and rechromatography (not shown). Computer fitting of the data by this minimal mechanism revealed that the prelatent species is formed from native antithrombin at a rate comparable with that of the latent species, but it subsequently reacts to form latent and non-heparin-binding forms at a much faster rate than it is itself formed.
Attempts to increase the formation of the prelatent species by an alternative heat treatment of native antithrombin in the presence of glycerol rather than citrate (19), by treatment with low concentrations of guanidine hydrochloride (36), or by incubation at 60 °C in the absence or presence of pentasaccharide or full-length heparins resulted in little or no detectable prelatent antithrombin. However, heating in the presence of high concentrations of anions previously shown to stabilize antithrombin during pasteurization (26), including 0.25, 0.5, or 1 m sodium citrate, 0.5 m sodium phosphate, 0.5 m sodium EDTA, or 3 m NaCl, all resulted in the formation of prelatent antithrombin, with 1 m sodium citrate producing the highest yield. Interestingly, incubation in 0.5 or 1 m citrate at a higher pH of 9.0 doubled the yield of prelatent antithrombin relative to that at pH 7.4, whereas similar incubation at a lower pH of 6 reduced the yield. The conversion of antithrombin to the prelatent species therefore requires stabilizing anions of the Hofmeister series (25) and is more favorable at higher pH. Although prelatent antithrombin converts more rapidly to the latent form than native antithrombin at 60 °C, the purified prelatent species is reasonably stable, since minimal transformation (<10%) was found to occur when incubated under physiologic conditions of I 0.15, pH 7.4, 5 mm CaCl2, and 37 °C for up to 48 h.
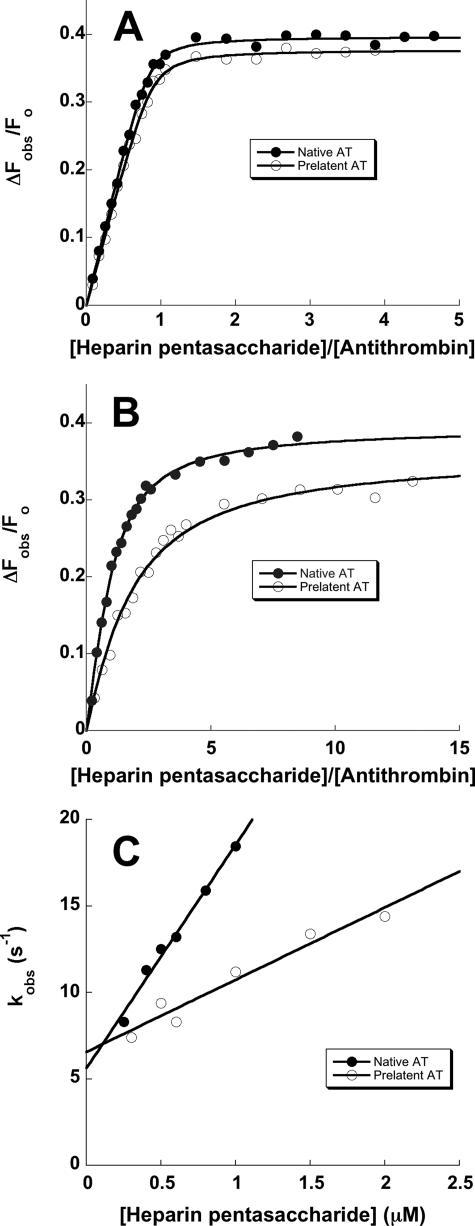
Binding of Heparin to Prelatent Antithrombin—The interaction of heparin with prelatent and native antithrombins was examined by equilibrium binding titrations with the high affinity pentasaccharide and a full-length heparin containing the pentasaccharide (7). These titrations were monitored by the tryptophan fluorescence enhancement that accompanies heparin binding and that reports conformational activation of the serpin. Whereas starting fluorescence intensities were indistinguishable for the two forms of antithrombin, pentasaccharide and full-length heparins induced similar but not equivalent tryptophan fluorescence enhancements of 34–35% in prelatent antithrombin and 39–42% in native antithrombin (Table 2). Prelatent antithrombin thus appeared to undergo an activating conformational change like that of native antithrombin upon binding the pentasaccharide. Titrations done at low ionic strength yielded stoichiometric binding curves indicative of a 1:1 heparin binding stoichiometry for both antithrombins, indicating that prelatent antithrombin was competent to bind heparin like the native serpin (Fig. 5A). However, titrations done at physiologic ionic strength and at concentrations approximating KD showed that the fitted KD values for pentasaccharide as well as full-length heparin binding were consistently ∼2-fold weaker for prelatent antithrombin than for native antithrombin (Fig. 5B, Table 2), in keeping with the reduced heparin affinity of the prelatent species observed by chromatography on heparin-agarose. For either native or prelatent antithrombins, full-length heparin bound with ∼3-fold higher affinity than the pentasaccharide (Table 2), suggesting that the extended heparin binding site was unperturbed in both antithrombin forms (7, 42). Binding studies at I 0.3 showed that the binding of pentasaccharide to both forms of antithrombin was weakened similarly by ∼15-fold (Table 2), implying similar ionic contributions to the binding of heparin to both forms of antithrombin but differing nonionic contributions.
FIGURE 5.
Comparison of the kinetics and affinity of the interaction of heparin with native and prelatent antithrombins. A and B compare representative fluorescence titrations of native (•) and prelatent (○) forms of antithrombin (AT) with the heparin pentasaccharide performed under stoichiometric binding conditions (I 0. 05) (A) or under equilibrium binding conditions (I 0.15) (B) as described under “Experimental Procedures.” Titrations were fit by the quadratic binding equation (solid lines) to obtain values for the binding stoichiometry, KD, and the maximal fluorescence change (Table 2). The fitted stoichiometry for the titration at I 0.05 was fixed in fitting the titration at I 0.15. C compares the kinetics of pentasaccharide binding to native (•) and prelatent (○) antithrombins under pseudo-first order conditions as a function of the pentasaccharide concentration. Solid lines are linear regression fits of data from which kon and koff were determined from the slope and intercepts, respectively.
Rapid kinetic analyses of the binding of pentasaccharide to native and prelatent antithrombins was done to determine whether the binding defect in prelatent antithrombin was due to a decreased on-rate constant (kon) or increased off-rate constant (koff). The binding kinetics were monitored by protein fluorescence changes under pseudo-first order conditions as a function of the pentasaccharide concentration and at I 0.3 to more accurately define the off-rate constant (7, 29). The observed pseudo-first order binding rate constant showed the expected linear dependence on pentasaccharide concentration for both antithrombins, the slopes yielding kon values of 12.9 ± 0.7 and 4.2 ± 0.5 μm-1 s-1 and the intercepts yielding koff values of 5.6 ± 0.5 and 6.6 ± 0.6 for native and prelatent antithrombin interactions, respectively (Fig. 5C). The weaker binding of heparin to prelatent antithrombin than to native antithrombin therefore arises primarily from a decreased kon.
Conformational Activation of Prelatent Antithrombin by High Affinity Heparin—Heparin induces an activating conformational change in antithrombin, which generates new exosites for promoting the inhibition of factor Xa and factor IXa (1, 8, 10, 11). To determine whether conformational activation of prelatent antithrombin exposes the same exosites as in native antithrombin, we tested the ability of heparin to promote the reaction of prelatent antithrombin with factor Xa. In the absence of heparin, prelatent antithrombin inhibited factor Xa with a similar rate constant as native antithrombin, consistent with the protein being predominantly in the unactivated state incapable of engaging factor Xa in exosite interactions (Table 1). Both pentasaccharide and full-length high affinity heparins accelerated the inhibition of factor Xa by prelatent antithrombin to an extent similar to that of native antithrombin at levels of heparin sufficient to saturate the two antithrombins (Fig. 6 and Table 1). Fitting of the full-length heparin concentration dependence of this rate enhancement confirmed the difference in KD values for heparin binding to the two forms of antithrombin that was observed in direct binding studies (see fitted KAT,H values in Table 3). The conformational activation of prelatent antithrombin by heparin therefore appeared to make the same exosites available for factor Xa interaction as with native antithrombin.
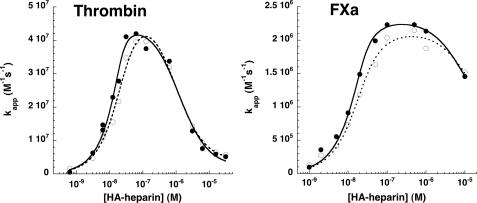
FIGURE 6.
Comparison of high affinity heparin-catalyzed reactions of native and prelatent antithrombin with proteases. Native (•) or prelatent (○) antithrombins (∼20 nm) were reacted with 1 nm thrombin (left) or 2 nm factor Xa (right) in the presence of variable concentrations of high affinity heparin (HA-heparin) for fixed times of 2 min (thrombin) or 30 s (factor Xa). Thrombin reactions additionally contained 2 mm p-aminobenzamidine to slow the rate sufficiently to allow accurate kinetic measurements. Apparent second order rate constants (kapp) were obtained by dividing observed pseudo-first order rate constants for protease inactivation by the functional antithrombin concentration. Additional corrections were made for the competitive effect of p-aminobenzamidine in reactions with thrombin. The bell-shaped heparin concentration dependence of kapp was fit by the ternary complex model (solid lines for native antithrombin, dashed lines for prelatent antithrombin) to provide values for the binary protein-heparin complex dissociation constants and the true second order rate constants for the reactions of antithrombin-heparin binary complex with each protease (32). These values are tabulated in Table 3. Further details are provided under “Experimental Procedures.”
TABLE 3.
Kinetics of heparin-catalyzed reactions of native and prelatent antithrombins with thrombin and factor Xa Kinetic parameters were obtained from fits of the data of Figs. 6 and 7 for high affinity heparin and low affinity heparin-catalyzed reactions of native and prelatent antithrombins with thrombin or factor Xa by the ternary complex bridging or conformational activation models described under “Experimental Procedures.” The fitted parameters were the rate constant for the reaction of heparin-complexed antithrombin with protease (kH) and the dissociation constants for the binary antithrombin-heparin complex (KAT,H) and the protease-heparin binary complex (KPr,H).
| Antithrombin | Protease | kH | KAT,H | KPr,H |
|---|---|---|---|---|
| m–1 s–1 | m | m | ||
| High affinity heparin-catalyzed reactions | ||||
| Native | IIa | 4.5 ± 0.3 × 107 | 2.0 ± 1.2 × 10–9 | 1.2 ± 0.4 × 10–6 |
| Prelatent | IIa | 5.0 ± 0.4 × 107 | 11 ± 3 × 10–9 | 0.91 ± 0.25 × 10–6 |
| Native | FXa | 2.3 ± 0.6 × 106 | 3.5 ± 1.0 × 10–9 | 10 ± 2 × 10–6 |
| Prelatent | FXa | 2.1 ± 0.8 × 106 | 8.4 ± 2.0 × 10–9 | 15 ± 5 × 10–6 |
| Low affinity heparin-catalyzed reactions | ||||
| Native | IIa | 6.4 ± 0.3 × 105 | 12 ± 2 × 10–5 | 3.6 ± 0.6 × 10–7 |
| Prelatent | IIa | 7.0 ± 0.2 × 105 | 8.5 ± 0.9 × 10–5 | 3.5 ± 0.3 × 10–7 |
| Native | FXa | 5.4 ± 0.2 × 105 | 2.2 ± 0.2 × 10–5 | |
| Prelatent | FXa | 4.0 ± 0.3 × 105 | 1.7 ± 0.3 × 10–5 |
The heparin-induced conformational change in antithrombin minimally affects the rate of thrombin inhibition (7). Heparin instead activates antithrombin to inhibit thrombin by an alternative bridging mechanism in which heparin provides a site for thrombin to bind next to antithrombin in a ternary complex that promotes the interaction of thrombin with antithrombin (13, 14). High affinity heparin accelerated the reactions of prelatent and native antithrombin with thrombin with similar bell-shaped dependences of the accelerating effect on heparin concentration that are diagnostic of the ternary complex bridging mechanism (35) (Fig. 6). Fitting of the bell-shaped curves revealed that heparin accelerated the reactions of both antithrombins to similar maximal extents, indicating that the ternary complex bridging mechanism of heparin promotion of the antithrombin-thrombin reaction was unaffected by the conversion of antithrombin to the prelatent species. As with the factor Xa reaction, the titration of the heparin rate enhancement confirmed a weaker affinity of prelatent antithrombin than of native antithrombin for heparin (Table 3).
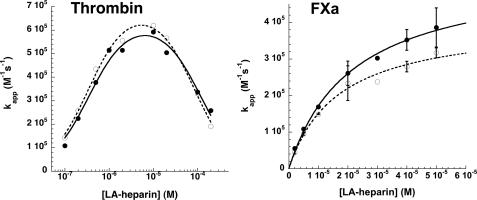
Activation of Prelatent Antithrombin by Low Affinity Heparin— High affinity heparin binding to antithrombin strongly favors the conformationally activated state of the inhibitor, whereas the binding of low affinity heparin lacking the pentasaccharide produces an equilibrium mixture of native and activated states and consequent reduced activating effect on the serpin (35). To determine whether the conformational activation effect of low affinity heparin might differ for prelatent and native forms of antithrombin, the kinetics of antithrombin inhibition of factor Xa was examined as a function of the low affinity heparin concentration. The apparent second order rate constant (kapp) increased in a saturable manner with increasing heparin concentration, consistent with previous findings demonstrating that this rate increase reflects the saturation of antithrombin with heparin (32) (Fig. 7). The apparent KD for the binding was not significantly different for the two forms of antithrombin, whereas the maximum rate constants at saturation significantly differed by a factor of ∼1.4 (Table 3). These results implied that low affinity heparin has a reduced ability to conformationally activate prelatent antithrombin compared with native antithrombin, in keeping with the conformational equilibrium of the low affinity heparin-bound inhibitor favoring the unactivated state more for the prelatent species than for the native species. Contrasting these results, a comparison of the activating effects of low affinity heparin on the reactions of prelatent and native antithrombin with thrombin showed that low affinity heparin bridging was similar for the two species of antithrombin (Fig. 7 and Table 3).
FIGURE 7.
Comparison of low affinity heparin-catalyzed reactions of native and prelatent antithrombins with proteases. Native (•) and prelatent (○) antithrombins were reacted with thrombin (20 nm inhibitor and 1 nm protease) or with factor Xa (50 nm inhibitor and 5 nm protease) in the presence of increasing concentrations of low affinity heparin (LA-heparin) for fixed times of 5 min or for variable reaction times. Apparent second order inactivation rate constants were calculated from observed pseudo-first order rate constants and the functional inhibitor concentration as in Fig. 6. The heparin concentration dependence of kapp was fit by the ternary complex bridging model for reactions with thrombin or by a model in which conformational activation of antithrombin solely contributed to the rate-enhancing effect of heparin for reactions with factor Xa (32). Solid lines indicate the fit of the native antithrombin kinetic data, and dashed lines indicate the fit of the prelatent antithrombin kinetic data.
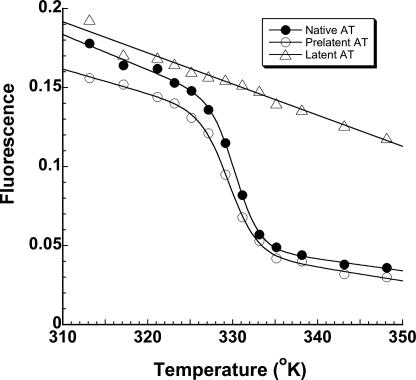
Conformational Stability of Prelatent Antithrombin—To assess the extent of conformational similarity of prelatent antithrombin to native and latent forms of the serpin, we compared the melting temperatures of these forms using protein fluorescence changes to monitor the melting transition. Prelatent antithrombin underwent a melting transition over a temperature range similar to that of native antithrombin, whereas latent antithrombin showed no melting transition over the range of temperatures examined (up to 75 °C) (Fig. 8). Fitting of the transitions gave a reproducible melting temperature for prelatent antithrombin of 56.8 ± 0.1 °C that was about 1 °C lower than the value of 57.7 ± 0.2 °C for native antithrombin in three separate experiments, a difference that was statistically significant (p = 0.015). The value for native antithrombin agrees with previous reports (43). Prelatent antithrombin therefore appears to be slightly less stable than native antithrombin.
FIGURE 8.
Decreased thermal stability of prelatent antithrombin. Shown are melting curves for native (•), prelatent (○), and latent (▵) forms of antithrombin measured from decreases in intrinsic protein fluorescence as a function of increasing temperature (in degrees Kelvin). Data were fit by the van't Hoff equation for a two-state unfolding transition to obtain the melting temperature corresponding to the midpoint of the unfolding curves, as described under “Experimental Procedures.” Fitted melting temperatures are reported in degrees centigrade. The results are representative of three independent experiments.
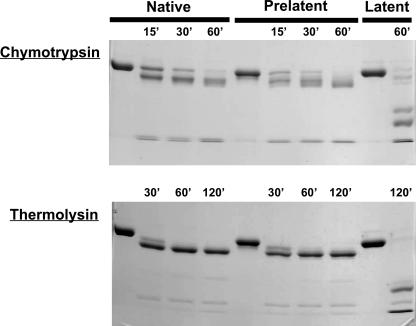
Susceptibility of Prelatent Antithrombin to Proteolysis—Previous studies suggested that prelatent antithrombin has an altered susceptibility to proteolysis by nontarget proteases compared with native antithrombin (24). To confirm this result, we compared the susceptibility of native, prelatent, and latent species to proteolysis by chymotrypsin and thermolysin, proteases examined in the previous study (Fig. 9). The pattern of proteolysis of prelatent antithrombin paralleled that of native antithrombin but differed from that of latent antithrombin. Both native and prelatent antithrombins showed a limited cleavage within 1 h similar to that shown previously to be produced by neutrophil elastase (32) without any further cleavage over 2 h. These findings are consistent with the cleavages previously shown to occur in the reactive loop and in the N-terminal region of both forms (24). By contrast, latent antithrombin showed a distinct pattern of cleavage to fragments with much lower molecular mass of 30–40 kDa (Fig. 9), in agreement with past studies (17). This latter pattern was similar to that previously reported for the proteolytic susceptibility of prelatent antithrombin (24), suggesting that the previous findings may have resulted from some latent antithrombin in the prelatent inhibitor fraction.
FIGURE 9.
Analysis of conformational alterations in prelatent antithrombin by proteolytic susceptibility. The susceptibility of native, prelatent, and latent forms of antithrombin to digestion by catalytic levels of the nontarget proteases, chymotrypsin and thermolysin (serpin/protease weight ratio of 10:1), was monitored as a function of digestion time by SDS-PAGE as described under “Experimental Procedures.”
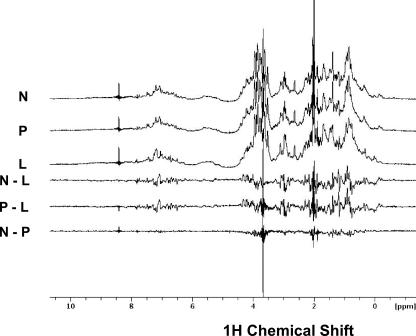
Analysis of Structural Differences between Native and Prelatent Antithrombins—To determine whether we could detect differences in the structure of native, prelatent, and latent antithrombins, we compared 1H NMR spectra of the three antithrombin forms, since such spectra provide fingerprints of structure (35, 44). The spectra of native and prelatent antithrombins showed a high degree of similarity, whereas both spectra showed marked differences from the spectrum of latent antithrombin (Fig. 10). Analysis of difference spectra confirmed that native and prelatent antithrombins exhibited highly similar protein structures.
FIGURE 10.
Conformational alterations in prelatent antithrombin probed by 1H NMR spectroscopy. Comparison of 900 MHz 1H NMR spectra of 10 μm samples of native (N), prelatent (P), and latent (L) antithrombins in I 0.15, pH 7.4, D2O buffer at 25 °C. Samples were prepared, and spectra were recorded as described under “Experimental Procedures.” Difference spectra between native and prelatent, native and latent, and prelatent and latent are shown to emphasize the similarity of native and prelatent spectra and the significant differences between either of these forms and latent antithrombin. The spike at 3.7 ppm represents small differences in the H2O content of the samples.
Relative Antiangiogenic Activities of Prelatent and Latent Antithrombins—The relative antiangiogenic potencies of prelatent and latent antithrombins relative to native antithrombin were tested by analyzing the dose dependence of the antiproliferative activities of the conformationally distinct antithrombins in an FGF-2-stimulated HUVEC proliferation assay. Prelatent antithrombin showed a more pronounced antiproliferative activity than latent antithrombin at equivalent doses, whereas native antithrombin showed insignificant antiproliferative effects (Fig. 3). Statistically significant inhibition of cell growth (p < 0.01) was evident at a 5-fold lower dose for prelatent antithrombin (10 μg/ml) than for latent antithrombin (50 μg/ml). The greater potency of prelatent versus latent antithrombin in this assay together with the demonstrated stability of the prelatent species over the 48-h time frame of this assay clearly showed that the antiproliferative effect is an intrinsic property of the prelatent form and cannot be ascribed to any transformation to the latent form during the assay. Prelatent antithrombin is thus a more potent antiangiogenic agent than latent antithrombin, in keeping with previous findings (24). Based on the relative antiangiogenic effects of latent and cleaved antithrombins (16), it is also likely to be more antiangiogenically active than cleaved antithrombin.
DISCUSSION
We have confirmed the existence of a novel conformational state of antithrombin termed the prelatent state that possesses a potent antiangiogenic activity and retains anticoagulant activity, as reported previously (24). However, our findings suggest that what was characterized in past studies as prelatent antithrombin was actually a mixture of the antiangiogenically active prelatent form and the native form of the serpin that lacks antiangiogenic activity. The prelatent fraction isolated as previously described is thus chromatographically resolvable into two distinct species differing in heparin affinity, as suggested by a recent study (45). The difference in heparin affinity is not large (i.e. about 2-fold), possibly accounting for the inability to resolve the prelatent antithrombin from native antithrombin in previous studies that monitored the protein eluted during heparin-agarose chromatography in discrete fractions rather than by continuous fluorescence monitoring as done in the present study. A convex salt gradient was used to decrease the steepness of the gradient in the range where the two species eluted, improving their chromatographic resolution. The prelatent and native species were clearly distinguishable based on their elution positions upon rechromatography and the finding that only the prelatent species possessed antiangiogenic activity as judged from the ability to inhibit FGF-2-stimulated growth of endothelial cells. The two antithrombin forms were shown to exhibit indistinguishable mobilities by native PAGE or SDS-PAGE and to form stoichiometric complexes with clotting proteases. However, we were able to show that the reactions of the two antithrombin forms with thrombin and factor Xa were accelerated by high affinity heparin species containing a pentasaccharide activating sequence to differing extents that were accounted for by their different affinities for heparin.
More revealing differences were exhibited when low affinity heparin species were employed to activate the two forms of the serpin. The prelatent species was thus not as conformationally activable by low affinity heparin as native antithrombin, as judged from the reduced ability of the activated prelatent antithrombin to enhance the inactivation of factor Xa. Low affinity heparin differs from high affinity heparin in binding antithrombin with ∼1000-fold reduced affinity and in failing to induce full conformational activation of the serpin (35). This results from the equilibrium between native and activated conformational states being balanced between the two states when low affinity heparin, which lacks a critical 3-O-sulfate, is bound to the serpin. By contrast, the equilibrium overwhelmingly favors the activated conformation when high affinity heparin is bound (6, 46, 47). Effects on the conformational activation equilibrium are thus most evident when the serpin is activated by low affinity heparin. The observations that the reduced affinity of prelatent antithrombin for the heparin pentasaccharide arises from a reduced nonionic contribution to the binding energy and a reduced association rate constant would be compatible with a perturbation of the conformational activation step, since the initial binding step is principally driven by electrostatics (29, 48). Thus, although prelatent antithrombin retains anticoagulant activity, the activity is reduced compared with native antithrombin due to a reduced affinity for high affinity heparin species and to a reduced conformational activation that is evident with low affinity heparin species. At the same time, the antiangiogenic activity of the prelatent species is more potent than either latent or cleaved antithrombin forms. The functional properties of prelatent antithrombin therefore clearly distinguish this species from all other known forms of antithrombin.
The conformational differences between native and prelatent antithrombins were probed by various means. The fluorescence properties of prelatent antithrombin were somewhat different in showing reduced fluorescence in the heparin-activated state, consistent with the environment of tryptophans being affected. 1H NMR spectra failed to show significant differences in the structures of prelatent and native antithrombins, in contrast to the more marked differences observed between native and latent antithrombins. The latter differences are consistent with the global conformational change accompanying the conversion of the native to the latent species. The native metastable state of antithrombin is characterized by a partial insertion of the reactive loop hinge into sheet A and conformational activation by heparin results in expulsion of the loop from the sheet (18, 42, 49). The melting temperature of prelatent antithrombin was lower than that of native antithrombin, suggesting that the conformational alteration in the prelatent state does not involve further insertion of the reactive loop into sheet A, since such insertion would have increased rather than decreased stability (49). We were unable to reproduce the finding of the past work that prelatent antithrombin underwent a unique cleavage in the C-terminal region between residues 325 and 375 by nontarget proteases (24). Rather, native and prelatent forms were found to show the same susceptibility to chymotrypsin and thermolysin cleavage, presumably involving cleavage in the N terminus and within the reactive loop, as shown previously for native antithrombin. Interestingly, a cleavage similar to that reported for the prelatent species in the past study was observed to occur with latent antithrombin, suggesting that some of the prelatent antithrombin in the past study may have converted to the latent species to account for this discrepancy. Prelatent antithrombin thus appears to strongly resemble native antithrombin in its overall conformation, although clearly differing in functional properties that must involve subtle differences in structure or chemical changes that affect heparin activation. Preliminary mass spectrometry analyses of endo-Lys-C-digested samples of the two antithrombins has shown no detectable chemical differences, although incomplete sequence coverage was obtained in these initial analyses.
Significantly, kinetic analysis of the formation of prelatent antithrombin from the native serpin and the observation that the isolated prelatent species converts to the latent form when it is returned to the conditions employed for its formation revealed that prelatent antithrombin is an intermediate in the conversion of native to latent antithrombin. Prelatent antithrombin may thus resemble the proposed prelatent intermediate in the conversion of native to latent plasminogen activator inhibitor-1 (27). This intermediate was suggested to involve a partial disruption of the interactions of strand 1C in the C-terminal reactive loop hinge with sheet C to allow the N-terminal end of the loop to more facilely insert into sheet A. Such a structure would be reconcilable with the reduced stability of prelatent antithrombin and its faster conversion to the latent form and be consistent with the properties of the proposed intermediate in the conversion of native antithrombin to the latent form (17). Moreover, this structure could account for the reduced heparin affinity of prelatent antithrombin and the decreased activating effect of low affinity heparin on the prelatent form if there was a greater energetic price to pay for converting the prelatent structure to a conformationally activated state. It should be noted that several published x-ray structures of antithrombin were determined by cocrystallization with latent antithrombin that had been prepared by heating in citrate buffer, with no reported evidence of chemical changes in the latent species (42, 50). This observation supports the view that prelatent antithrombin is a structurally altered intermediate on the pathway to latent antithrombin and not the result of chemical alterations.
Most interesting was the finding that formation of prelatent antithrombin depended on the presence of anions that are known to stabilize antithrombin activity during pasteurization of the protein for clinical use (26). The stabilizing effects of the anions correlate well with their position in the Hofmeister series and result from their enhancing intramolecular hydrophobic interactions. These effects delay the intermolecular polymerization of antithrombin through reactive loop-A sheet interactions and favor an intramolecular insertion of the reactive loop into sheet A to form the latent species. The anions thus transform antithrombin into a plasminogen activator inhibitor-1-like serpin, which normally prefers intramolecular insertion of the reactive loop into sheet A through a prelatent intermediate over an intermolecular insertion that results in polymers. Based on our findings, it would appear that the conversion to the prelatent form commits the protein to forming the much more stable latent form, since reversion to the native species was not observed. Moreover, alternate pathways to the latent state, possibly involving the disruption of the s3C-s4C steric “gate” that obstructs the insertion of the reactive loop into sheet A, are likely to exist, since the prelatent state was not an obligate intermediate in the conversion to the latent state.
Our results suggest that the limited structural changes involved in forming prelatent antithrombin expose an epitope required for antiangiogenic activity. If these changes involve a limited disruption of the s1C interaction, then the epitope may reside in the region exposed by detachment of s1C (27). The finding that prelatent antithrombin has a greater antiangiogenic potency than latent antithrombin suggests that this epitope is more accessible or in a more optimal conformation in prelatent than in latent or cleaved forms of antithrombin. Our previous studies suggested that such an epitope also existed in the unactivated native antithrombin conformation in that a mutant antithrombin defective in heparin binding and unable to undergo conformational activation was antiangiogenically active without the need for conversion to latent or cleaved forms (31). Since the native antithrombin conformation is characterized by insertion of the N-terminal reactive loop hinge into sheet A, it is possible that this insertion could disrupt the s1C interaction in the C-terminal reactive loop hinge and expose the antiangiogenic epitope. Heparin activation expels the reactive loop from sheet A and would be expected to restore the s1C interaction and account for the masking of the epitope in the native serpin. An x-ray structure of prelatent antithrombin will be necessary to provide further insights into the structural changes in antithrombin that are responsible for the expression of antiangiogenic activity.
Past studies have shown that prelatent and latent antithrombins are present in heat-treated commercial preparations of antithrombin (24), in keeping with such preparations having undergone pasteurization in the presence of stabilizing anions (26). Prelatent antithrombin thus is clinically significant in the treatment of patients with congenital or acquired deficiencies of antithrombin by replacement therapy. However, prelatent antithrombin is less likely to have physiologic significance given our finding that prelatent antithrombin was not detectable in the conversion of antithrombin to the latent form under more physiologic conditions. Latent and cleaved antithrombins therefore most likely account for the reported antiangiogenic effects of antithrombin in vivo (51). Nevertheless, the greater antiangiogenic potency of prelatent antithrombin over latent and cleaved forms and the conditions we have established for producing this species suggest potential clinical applications for antitumor therapy. This is underscored by the fact that the antiangiogenic activity of cleaved and latent forms of antithrombin is already comparable with that of other well established angiogenesis inhibitors, such as endostatin and TNP-470 (16, 20).
Acknowledgments
We thank Richard Guerre and Bahareh Zyaie for technical assistance with some of the studies and Ingemar Björk for critical comments and helpful suggestions on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants HL-39888 (to S. T. O.), HL-49234 (to P. G. W. G.), and P41 GM68944 (for the 900-MHz NMR spectrometer). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: MES, 4-morpholineethanesulfonic acid; TAPS, 3-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}-1-propanesulfonic acid; HUVECs, human umbilical vein endothelial cells; FGF-2, human fibroblast growth factor-2.
References
- 1.Olson, S. T., and Chuang, Y.-J. (2002) Trends Cardiovasc. Med. 12 331-338 [DOI] [PubMed] [Google Scholar]
- 2.Olson, S. T., Björk, I., and Bock, S. C. (2002) Trends Cardiovasc. Med. 12 198-205 [DOI] [PubMed] [Google Scholar]
- 3.Church, F. C., Pike, R. N., Tollefsen, D. M., Buckle, A. M., Ciaccia, A. V., and Olson, S. T. (2007) in Molecular and Cellular Aspects of the Serpinopathies and Disorders of Serpin Activity (Silverman, G. A., and Lomas, D. A., eds) pp. 509-554, World Scientific Publishing, Singapore
- 4.Huntington, J. A., Read, R. J., and Carrell, R. W. (2000) Nature 407 923-926 [DOI] [PubMed] [Google Scholar]
- 5.Dementiev, A., Dobó, J., and Gettins, P. G. W. (2006) J. Biol. Chem. 281 3452-3457 [DOI] [PubMed] [Google Scholar]
- 6.Choay, J., Petitou, M., Lormeau, J. C., Sinay, P., Casu, B., and Gatti, G. (1983) Biochem. Biophys. Res. Commun. 116 492-499 [DOI] [PubMed] [Google Scholar]
- 7.Olson, S. T., Björk, I., Sheffer, R., Craig, P. A., Shore, J. D., and Choay, J. (1992) J. Biol. Chem. 267 12528-12538 [PubMed] [Google Scholar]
- 8.Johnson, D. J., Li, W., Adams, T. E., and Huntington, J. A. (2006) EMBO J. 25 2029-2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izaguirre, G., Zhang, W., Swanson, R., Bedsted, T., and Olson, S. T. (2003) J. Biol. Chem. 278 51433-51440 [DOI] [PubMed] [Google Scholar]
- 10.Izaguirre, G., Swanson, R., Raja, S. M., Rezaie, A. R., and Olson, S. T. (2007) J. Biol. Chem. 282 33609-33622 [DOI] [PubMed] [Google Scholar]
- 11.Izaguirre, G., and Olson, S. T. (2006) J. Biol. Chem. 281 13424-13432 [DOI] [PubMed] [Google Scholar]
- 12.Olson, S. T., and Björk, I. (1991) J. Biol. Chem. 266 6353-6364 [PubMed] [Google Scholar]
- 13.Dementiev, A., Petitou, M., Herbert, J., and Gettins, P. G. W. (2004) Nat. Struct. Mol. Biol. 11 863-867 [DOI] [PubMed] [Google Scholar]
- 14.Li, W., Johnson, D. J. D., Esmon, C. T., and Huntington, J. A. (2004) Nat. Struct. Mol. Biol. 11 857-862 [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly, M. S., Pirie-Shepherd, S., Lane, W. S., and Folkman, J. (1999) Science 285 1926-1928 [DOI] [PubMed] [Google Scholar]
- 16.Larsson, H., Sjoblom, T., Dixelius, J., Ostman, A., Ylinenjärvi, K., Björk, I., and Claesson-Welsh, L. (2000) Cancer Res. 60 6723-6729 [PubMed] [Google Scholar]
- 17.Wardell, M. R., Chang, W. S. W., Bruce, D., Skinner, R., Lesk, A. M., and Carrell, R. W. (1997) Biochemistry 36 13133-13142 [DOI] [PubMed] [Google Scholar]
- 18.Skinner, R., Abrahams, J.-P., Whisstock, J. C., Lesk, A. M., Carrell, R. W., and Wardell, M. R. (1997) J. Mol. Biol. 266 601-609 [DOI] [PubMed] [Google Scholar]
- 19.Zhou, A., Huntington, J. A., and Carrell, R. W. (1999) Blood 94 3388-3396 [PubMed] [Google Scholar]
- 20.Prox, D., Becker, C., Pirie-Shepherd, S. R., Celik, I., Folkman, J., and Kisker, O. (2003) World J. Surg. 68 47-54 [DOI] [PubMed] [Google Scholar]
- 21.Dawson, D. W., Volpert, O. V., Gillis, P., Crawford, S. E., Xu, H., Benedict, W., and Bouck, N. P. (1999) Science 285 245-248 [DOI] [PubMed] [Google Scholar]
- 22.Zhang, M., Volpert, O., Shi, Y. H., and Bouck, N. (2000) Nat. Med. 6 196-199 [DOI] [PubMed] [Google Scholar]
- 23.Miao, R. Q., Agata, J., Chao, L., and Chao, J. (2002) Blood 100 3245-3252 [DOI] [PubMed] [Google Scholar]
- 24.Larsson, H., Akerud, P., Nordling, K., Raub-Segall, E., Claesson-Welsh, L., and Björk, I. (2001) J. Biol. Chem. 276 11996-12002 [DOI] [PubMed] [Google Scholar]
- 25.von Hippel, P. H., and Schleich, T. (1969) Acc. Chem. Res. 2 257-265 [Google Scholar]
- 26.Busby, T. F., Atha, D. H., and Ingham, K. C. (1981) J. Biol. Chem. 256 12140-12147 [PubMed] [Google Scholar]
- 27.Dupont, D. M., Blouse, G. E., Hansen, M., Mathiasen, L., Kjelgaard, S., Jensen, J. K., Christensen, A., Gils, A., Declerck, P. J., Andreasen, P. A., and Wind, T. (2006) J. Biol. Chem. 281 36071-36081 [DOI] [PubMed] [Google Scholar]
- 28.Olson, S. T., Björk, I., and Shore, J. D. (1993) Methods Enzymol. 222 525-560 [DOI] [PubMed] [Google Scholar]
- 29.Turk, B., Brieditis, I., Bock, S. C., Olson, S. T., and Björk, I. (1997) Biochemistry 36 6682-6691 [DOI] [PubMed] [Google Scholar]
- 30.Nordenman, B., Nyström, C., and Björk, I. (1977) Eur. J. Biochem. 78 195-203 [DOI] [PubMed] [Google Scholar]
- 31.Zhang, W., Swanson, R., Izaguirre, G., Xiong, Y., Lau, L. F., and Olson, S. T. (2005) Blood 106 1621-1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jordan, R. E., Kilpatrick, J., and Nelson, R. M. (1987) Science 237 777-780 [DOI] [PubMed] [Google Scholar]
- 33.Miletich, J. P., Broze, G. J., Jr., and Majerus, P. W. (1981) Methods Enzymol. 80 221-228 [Google Scholar]
- 34.Owen, W. G., and Jackson, C. M. (1973) Thromb. Res. 3 705-714 [Google Scholar]
- 35.Streusand, V. J., Björk, I., Gettins, P. G. W., Petitou, M., and Olson, S. T. (1995) J. Biol. Chem. 270 9043-9051 [DOI] [PubMed] [Google Scholar]
- 36.Fish, W. W., Danielsson, Å., Nordling, K., Miller, S. H., Lam, C. F., and Björk, I. (1985) Biochemistry 24 1510-1517 [DOI] [PubMed] [Google Scholar]
- 37.Lewis, S. D., Shields, P. P., and Shafer, J. A. (1985) J. Biol. Chem. 260 10192-10199 [PubMed] [Google Scholar]
- 38.Laemmli, U. K. (1970) Nature 227 680-685 [DOI] [PubMed] [Google Scholar]
- 39.Rezaie, A. R. (1998) J. Biol. Chem. 273 16824-16827 [DOI] [PubMed] [Google Scholar]
- 40.Lawrence, D. A., Olson, S. T., Palaniappan, S., and Ginsburg, D. (1994) Biochemistry 33 3643-3648 [DOI] [PubMed] [Google Scholar]
- 41.Edsall, J. T., and Gutfreund, H. (1984) Biothermodynamics: The Study of Biochemical Processes at Equilibrium, John Wiley & Sons, Inc., New York
- 42.Jin, L., Abrahams, J. P., Skinner, R., Petitou, M., Pike, R. N., and Carrell, R. W. (1997) Proc. Natl. Acad. Sci. U. S. A. 94 14683-14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Björk, I., Ylinenjärvi, K., Olson, S. T., and Bock, P. E. (1992) J. Biol. Chem. 267 1976-1982 [PubMed] [Google Scholar]
- 44.Gettins, P. (1987) Biochemistry 26 1391-1398 [DOI] [PubMed] [Google Scholar]
- 45.Karlsson, G., and Winge, S. (2004) Protein Expression Purif. 33 339-345 [DOI] [PubMed] [Google Scholar]
- 46.Lindahl, U., Bäckström, G., Thunberg, L., and Leder, I. G. (1980) Proc. Natl. Acad. Sci. U. S. A. 77 6551-6555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atha, D. H., Lormeau, J. C., Petitou, M., Rosenberg, R. D., and Choay, J. (1985) Biochemistry 24 6723-6729 [DOI] [PubMed] [Google Scholar]
- 48.Olson, S. T., Srinivasan, K. R., Björk, I., and Shore, J. D. (1981) J. Biol. Chem. 256 11073-11079 [PubMed] [Google Scholar]
- 49.Huntington, J. A., Olson, S. T., Fan, B., and Gettins, P. G. W. (1996) Biochemistry 35 8495-8503 [DOI] [PubMed] [Google Scholar]
- 50.Skinner, R., Chang, W.-S. W., Jin, L., Pei, X., Huntington, J. A., Abrahams, J.-P., Carrell, R. W., and Lomas, D. A. (1998) J. Mol. Biol. 283 9-14 [DOI] [PubMed] [Google Scholar]
- 51.Kisker, O., Onizuka, S., Banyard, J., Komiyama, T., Becker, C. M., Achilles, E. G., Barnes, C. M., O'Reilly, M. S., Folkman, J., and Pririe-Sheperd, S. R. (2001) Cancer Res. 61 7298-7304 [PubMed] [Google Scholar]