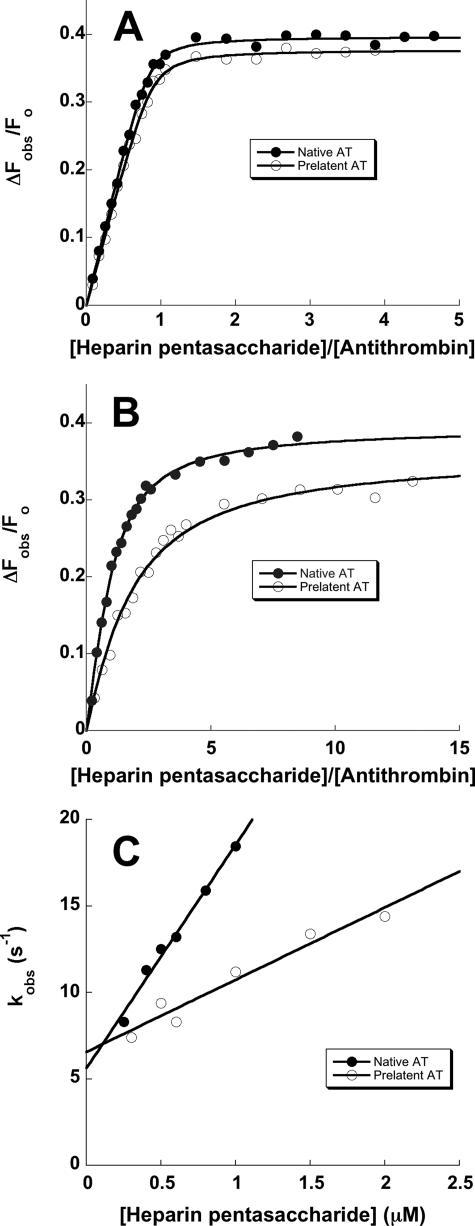
FIGURE 5.
Comparison of the kinetics and affinity of the interaction of heparin with native and prelatent antithrombins. A and B compare representative fluorescence titrations of native (•) and prelatent (○) forms of antithrombin (AT) with the heparin pentasaccharide performed under stoichiometric binding conditions (I 0. 05) (A) or under equilibrium binding conditions (I 0.15) (B) as described under “Experimental Procedures.” Titrations were fit by the quadratic binding equation (solid lines) to obtain values for the binding stoichiometry, KD, and the maximal fluorescence change (Table 2). The fitted stoichiometry for the titration at I 0.05 was fixed in fitting the titration at I 0.15. C compares the kinetics of pentasaccharide binding to native (•) and prelatent (○) antithrombins under pseudo-first order conditions as a function of the pentasaccharide concentration. Solid lines are linear regression fits of data from which kon and koff were determined from the slope and intercepts, respectively.