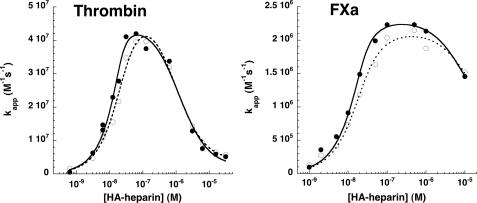
FIGURE 6.
Comparison of high affinity heparin-catalyzed reactions of native and prelatent antithrombin with proteases. Native (•) or prelatent (○) antithrombins (∼20 nm) were reacted with 1 nm thrombin (left) or 2 nm factor Xa (right) in the presence of variable concentrations of high affinity heparin (HA-heparin) for fixed times of 2 min (thrombin) or 30 s (factor Xa). Thrombin reactions additionally contained 2 mm p-aminobenzamidine to slow the rate sufficiently to allow accurate kinetic measurements. Apparent second order rate constants (kapp) were obtained by dividing observed pseudo-first order rate constants for protease inactivation by the functional antithrombin concentration. Additional corrections were made for the competitive effect of p-aminobenzamidine in reactions with thrombin. The bell-shaped heparin concentration dependence of kapp was fit by the ternary complex model (solid lines for native antithrombin, dashed lines for prelatent antithrombin) to provide values for the binary protein-heparin complex dissociation constants and the true second order rate constants for the reactions of antithrombin-heparin binary complex with each protease (32). These values are tabulated in Table 3. Further details are provided under “Experimental Procedures.”