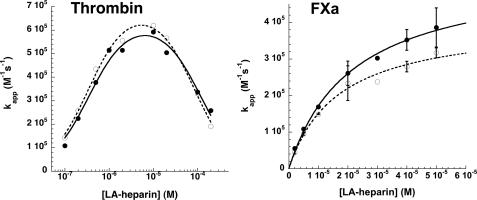
FIGURE 7.
Comparison of low affinity heparin-catalyzed reactions of native and prelatent antithrombins with proteases. Native (•) and prelatent (○) antithrombins were reacted with thrombin (20 nm inhibitor and 1 nm protease) or with factor Xa (50 nm inhibitor and 5 nm protease) in the presence of increasing concentrations of low affinity heparin (LA-heparin) for fixed times of 5 min or for variable reaction times. Apparent second order inactivation rate constants were calculated from observed pseudo-first order rate constants and the functional inhibitor concentration as in Fig. 6. The heparin concentration dependence of kapp was fit by the ternary complex bridging model for reactions with thrombin or by a model in which conformational activation of antithrombin solely contributed to the rate-enhancing effect of heparin for reactions with factor Xa (32). Solid lines indicate the fit of the native antithrombin kinetic data, and dashed lines indicate the fit of the prelatent antithrombin kinetic data.