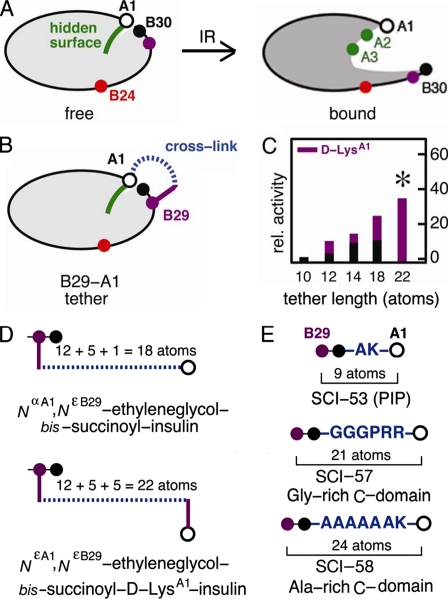
FIGURE 2.
Model of induced fit and design of interchain tethers. A, whereas the free conformation of insulin is closed (left panel), upon receptor binding, detachment of the C-terminal B-chain β-strand is proposed to expose the hidden non-polar surface of the A-chain (right panel), including IleA2 and ValA3 (green). The aromatic side chain of PheB24 (red) may function as a structural switch. GlyA1 is shown as an open circle, and B29 and B30 are shown as purple and back circles, respectively. B, tethering the A- and B-chains was effected by bifunctional cross-linking (dashed blue line) between the ε-amino group of LysB29 (purple circle) and the α-amino group of GlyA1 (open circle). C, a histogram showing the relative (rel.) activity of tethered insulin analogs is plotted as a function of the number of atoms between the α-carbons of B29 and A1 (horizontal axis), including the B29 side chain and, when present, the side chain of d-LysA1 (magenta). Bar heights indicate receptor-binding affinities relative to wild-type human insulin. Black bars indicate linkage to the α-amino group of GlyA1, whereas magenta bars indicate linkage to the ε-amino group of d-LysA1. The asterisk indicates substantial relative activity (35 ± 3%) exhibited by NεA1,NεB29-ethylene glycol bissuccinoyl d-LysA1-insulin. D, shown is a schematic design of representative cross-linked insulin derivatives. Upper panel, NαA1,NεB29-ethylene glycol bissuccinoyl insulin; lower panel, NεA1,NεB29-ethylene glycol bissuccinoyl d-LysA1-insulin. In the upper construct, the total number of atoms between the α-carbons of B29 and A1 is the sum of the lengths of the linker (dashed blue line; 12 atoms) and the LysB29 side chain (magenta; 5 atoms) plus the Nα of GlyA1:12 + 5 + 1 = 18. In the lower construct, the tether is extended by the side chain of d-LysA1:12 + 5 + 5 = 22. E, shown is the design of three single-chain analogs. Upper panel, PIP, a 53-residue analog (SCI-53) with a dipeptide-connecting domain (AK); middle panel, SCI-57; lower panel, 58-residue analog (SCI-58) with connecting domain AAAAAAK. In SCI-57, B30 (Thr) and a 6-residue linker (sequence GGGPRR) interpose 23 atoms between the α-carbons of B29 and A1; the corresponding numbers of intervening atoms in PIP and SCI-58 are 11 and 26, respectively.