Fig. 6.
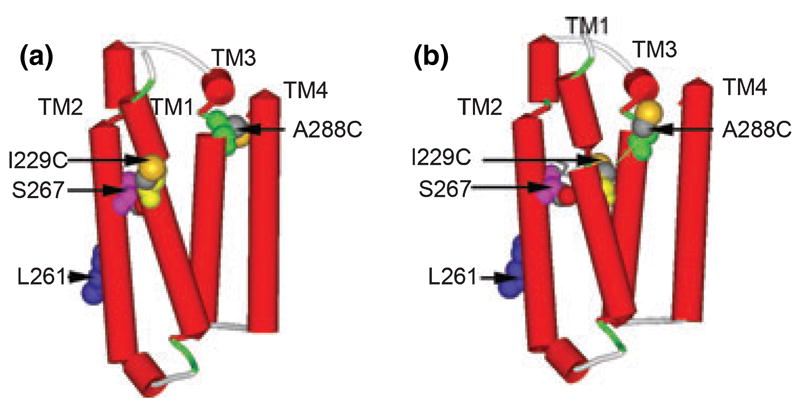
Molecular models of the TM domain of GlyRs. (a) The TM domain with the sequence of GlyR threaded directly onto the structure of nAChR (PDB ID 2BG9) with a previously suggested alignment (Bertaccini and Trudell 2002). The following residues were rendered with space filling surfaces: I229C is yellow, Leu261 (9′as a marker of a pore-lining residue) is blue, S267 is pink, A288C is green, and sulfur atoms are highlighted in orange. (b) The TM domain with the same residues after insertion of two gaps after Gly221 and one additional gap after Lys281. That is, GlyR Ile229 that was aligned with nAChR Ile220 in (a) is now aligned with Cys222 and GlyR A288 that was aligned with nAChR Leu279 in (a) is now aligned with Phe280. The thin green line shows the C-alpha to C-alpha dimension of 12.4 Å between I229C and A288C.
