Figure 5.
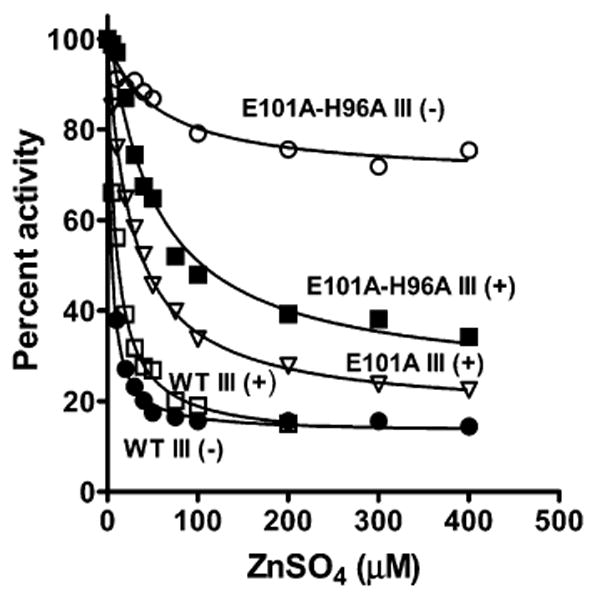
Inhibition of steady-state O2 reduction by zinc for wild-type CcO and different forms of the K pathway mutants in the presence of cholic acid. The activity of CcO was measured as described in Materials and Methods, in the presence of ZnSO4 at pH 7.4. All reactions included 1.1 mM cholic acid and 1 mg/mL soybean phospholipid (asolectin). The zinc inhibition constants were determined by nonlinear least-squares fitting as described by Mills et al. (24). Symbols and Ki values are as follows: WT III(−), 3.8 ± 0.2 μM (●); WT III(+), 9.3 ± 0.6 μM (□); E101A III(+), 28 ± 1.5 μM (▽); E101A/H96A III(+), 50 ± 9 μM (■); and E101A/H96A III(−), 56 ± 18 μM (○). The data for WT III(−) were taken from ref 19.
