Figure 1.
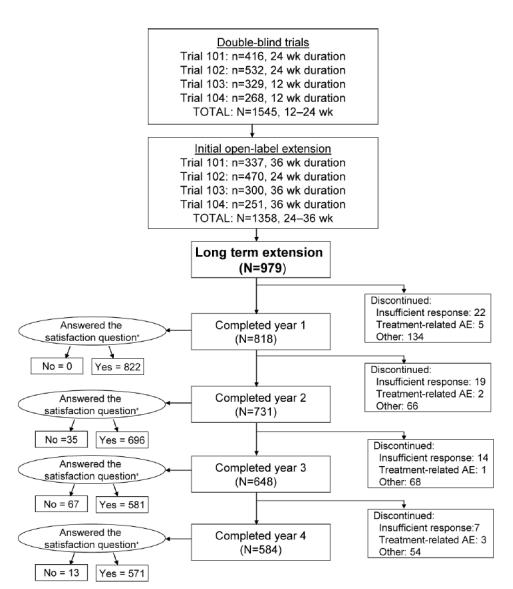
Number of patients and treatment duration in the original double-blind trials and the initial open-label extensions and disposition throughout 4 years of open-label, flexible-dose (25, 50, and 100 mg) sildenafil long-term extension study. Of the 979 participants who entered the sildenafil long-term extension study, 584 (60%) completed all 4 years. Reasons for discontinuation, other than insufficient clinical response and treatment-related adverse event (AE), included AE unrelated to treatment, protocol violation, loss to follow-up, and other (eg, loss of interest in participation in a clinical study, loss of sexual partner, and ability to afford sildenafil after approval [thus, no longer needing to participate in a clinical study to obtain the medication]). N for the satisfaction question (“Are you satisfied with the effect of treatment on your erections?”) is equal to the number of men who answered. *Some of the discontinued subjects supplied satisfaction data.
