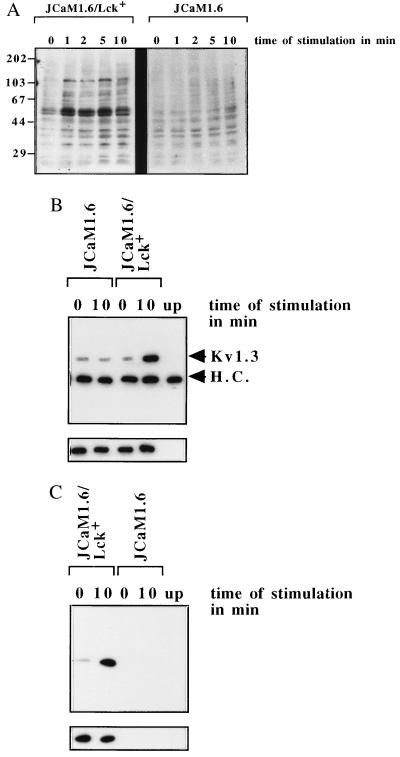
Figure 5.
(A) Genetic deficiency of p56lck prevents cellular tyrosine phosphorylation upon treatment with 10 μM C6-ceramide, whereas retransfection of p56lck restores the tyrosine phosphorylation of cellular proteins by C6-ceramide. JCaM1.6 or JCaM1.6/Lck+ cells were stimulated with C6-ceramide and lysed, and the whole-cell lysates were analyzed for tyrosine phosphorylation as above. (B) Tyrosine phosphorylation of the n-K+ channel by C6-ceramide depends on the functional expression of p56lck in JCaM1.6/Lck+ cells and is absent in p56lck-deficient JCaM1.6 cells. JCaM1.6/Lck+ or p56lck-deficient JCaM1.6 cells were stimulated with 10 μM C6-ceramide for the indicated time, and the n-K+ channel protein was immunoprecipitated and tested for tyrosine phosphorylation by Western blotting. The strips show similar amounts of Kv1.3 protein in all lanes (small blots). (C) The tyrosine kinase p56lck is stimulated by C6-ceramide in JCaM1.6/Lck+ cells, whereas no signal could be detected in the p56lck genetically deficient JCaM1.6 cells. Kinase activity was determined by autophosphorylation as above.