Fig. 3. G-CSF-responsive induction of αM and β2 integrin subunit expression is blocked by Stat3-DN.
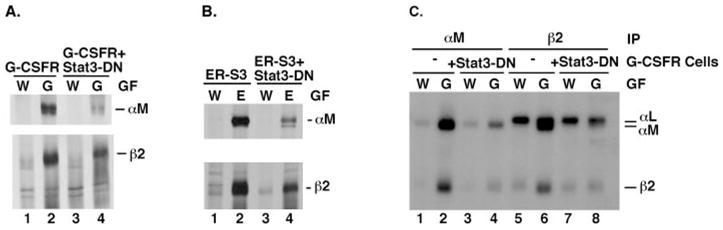
A, 32D.G-CSFR (G-CSFR) and 32D.G-CSFR + Stat3-DN (G-CSFR + Stat3-DN) cells were cultured in RPMI/FCS containing WEHI cell conditioned medium (W) or 25 ng/ml G-CSF (G) for 7 days. Proteins were metabolically labeled with [35S]methionine and cysteine and immunoprecipitated with antibodies specific for murine αM or β2 integrin subunits, as indicated. Polypeptides were resolved by SDS-PAGE and visualized by autoradiography. B, 32D.ER-S3 (ER-S3) and 32D.ER-S3 + Stat3-DN (ER-S3 + Stat3-DN) cells were cultured in RPMI/FCS containing WEHI cell conditioned medium (W) or 0.5 units/ml Epo (E) for 2 days. Proteins were metabolically labeled and analyzed by immunoprecipitations as described for A. C, 32D.G-CSFR cells, containing or lacking Stat3-DN (as indicated), were cultured in WEHI-containing (W) or G-CSF-containing (G) medium as described for A. Cells were radioiodinated, polypeptides were immunoprecipitated from detergent cell extracts with antibodies specific for murine αM or β2 integrin subunits (as indicated), and polypeptides were resolved by SDS-PAGE. The migration positions of αM (~170 kDa) and β2 (~95 kDa) integrin subunits are shown.
