Abstract
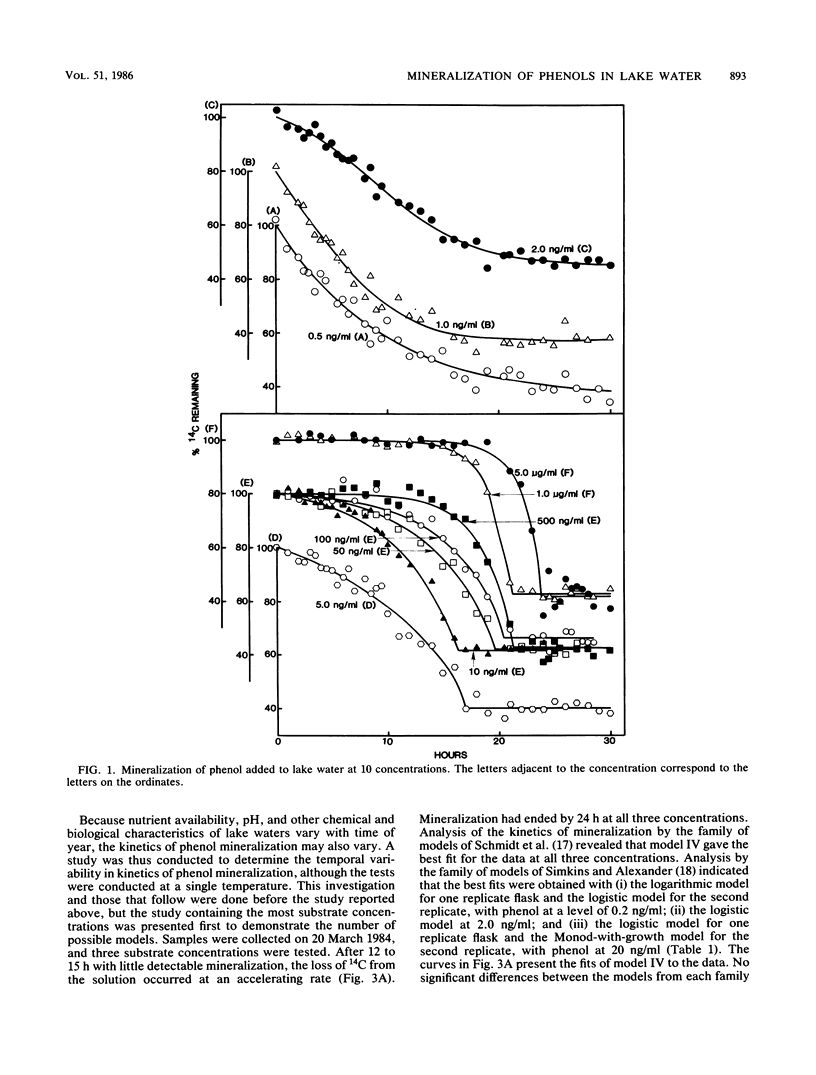
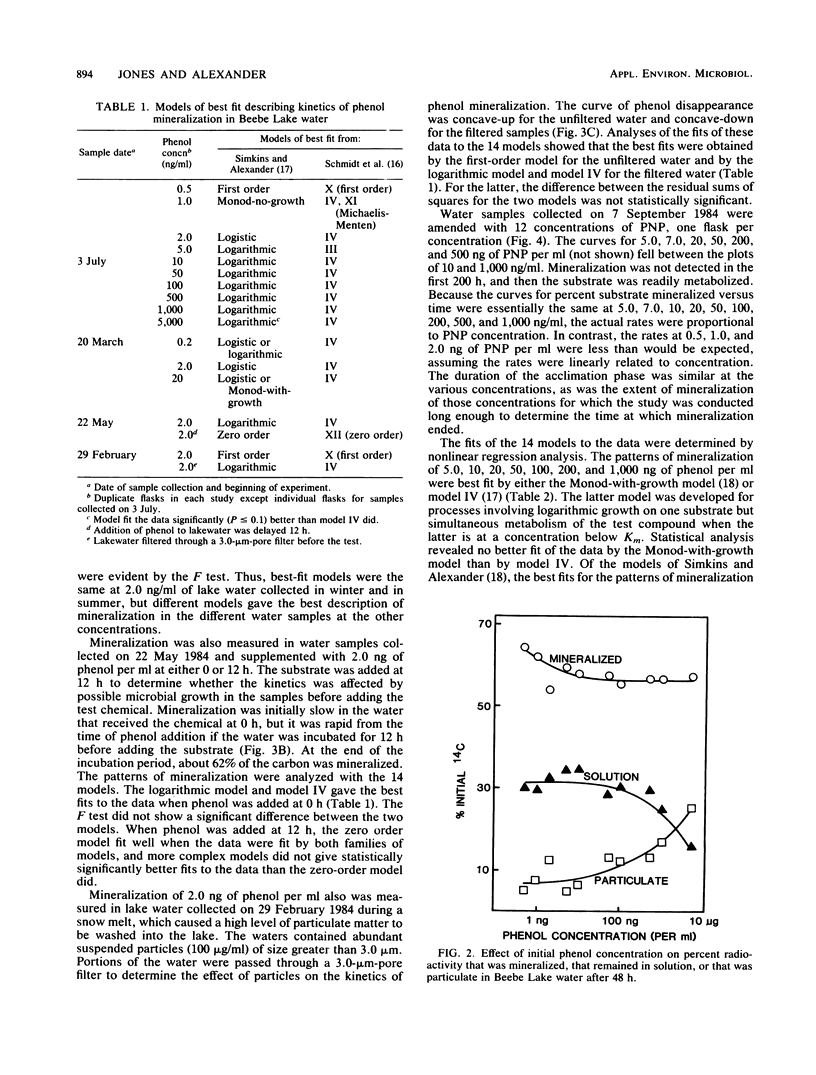
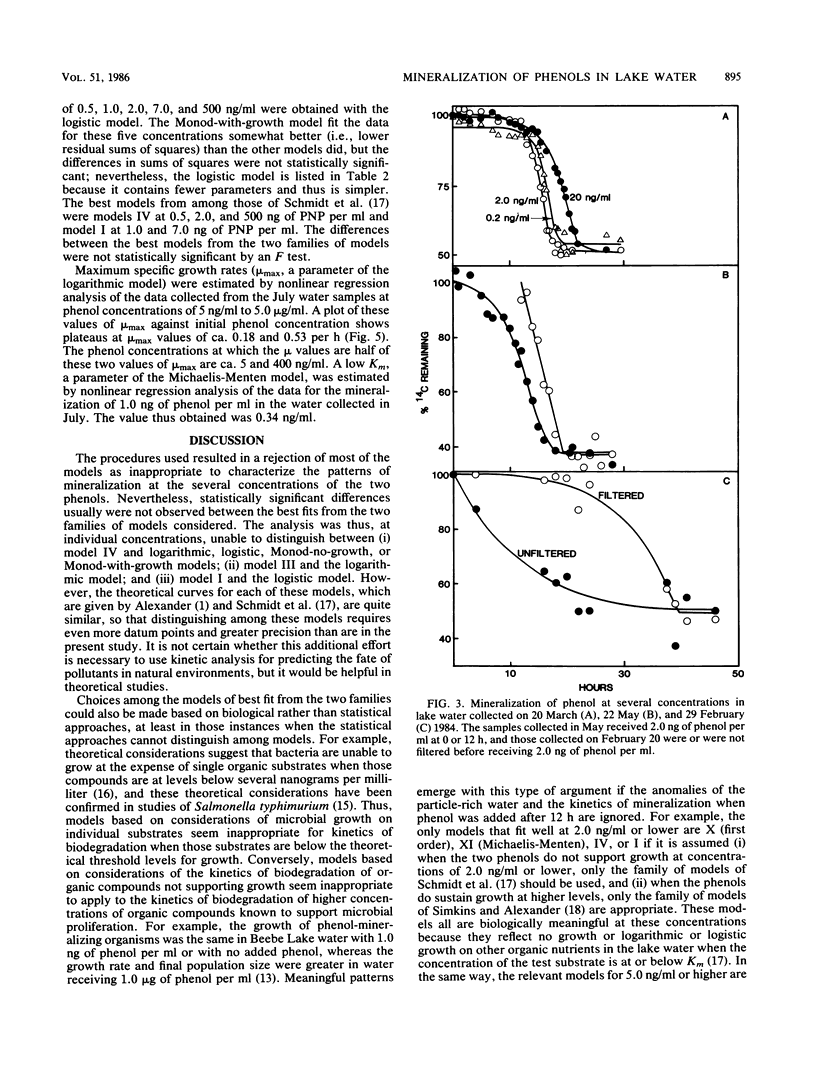
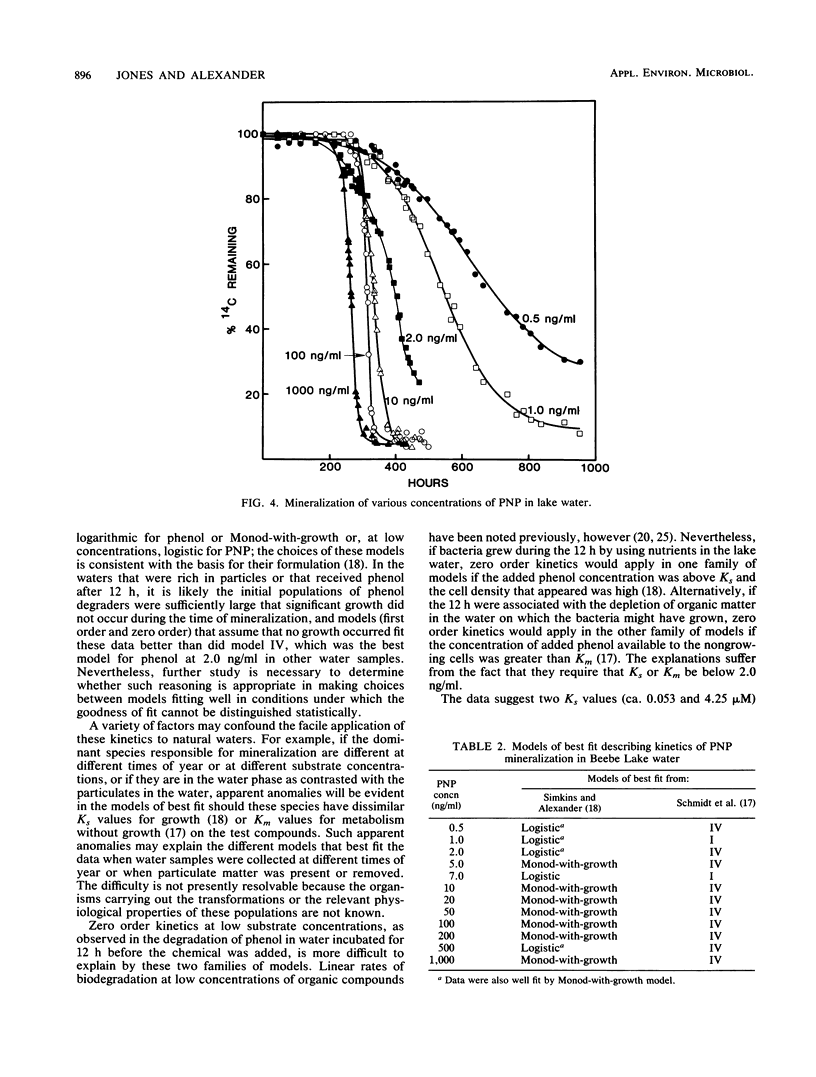
The kinetics of mineralization of phenol and p-nitrophenol in lake water was determined at concentrations from 200 pg/ml to 5 micrograms/ml. The mineralization data were fit by nonlinear regression to equations for 14 kinetic models that describe patterns of biodegradation by nongrowing cells or by microorganisms growing on either the test chemical or other organic substrates. The kinetics od mineralization of phenol in water samples collected in July was best described by first-order models for 0.5 ng of phenol per ml; by Monod-without-growth, logistic, and logarithmic models for 1.0 and 2.0 ng/ml and 5.0 ng/ml to 1.0 micrograms/ml, respectively, if it is assumed that the mineralizing population uses phenol as the sole carbon source for growth; by models (for phenol at concentrations of 2.0 ng/ml to 1.0 micrograms/ml) that assume that the phenol-mineralizing populations do not grow or grow logarithmically or logistically on uncharacterized carbon compounds but metabolize the phenol when present at levels below and above Km, respectively, for that compound; and by a logarithmic model at 5.0 micrograms/ml. Under the test conditions, usually less than 10% of the phenol C that was metabolized was incorporated into microbial cells or retained by other particulate material in the water at substrate concentrations of 10 ng/ml or less, and the percentage increased at higher substrate concentrations.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deeley G. M., Skierkowski P., Robertson J. M. Biodegradation of [14C]phenol in secondary sewage and landfill leachate measured by double-vial radiorespirometry. Appl Environ Microbiol. 1985 Apr;49(4):867–869. doi: 10.1128/aem.49.4.867-869.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. L., Hodson R. E., Freeman L. F. Multiphasic kinetics for transformation of methyl parathion by flavobacterium species. Appl Environ Microbiol. 1985 Sep;50(3):553–557. doi: 10.1128/aem.50.3.553-557.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm N., Lindgaard-Jørgensen P., Hansen N. Biodegradation of 4-nitrophenol in standardized aquatic degradation tests. Ecotoxicol Environ Saf. 1984 Oct;8(5):451–470. doi: 10.1016/0147-6513(84)90066-6. [DOI] [PubMed] [Google Scholar]
- Page T., Harris R. H., Epstein S. S. Drinking water and cancer mortality in Louisiana. Science. 1976 Jul 2;193(4247):55–57. doi: 10.1126/science.935854. [DOI] [PubMed] [Google Scholar]
- Paris D. F., Steen W. C., Baughman G. L., Barnett J. T. Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol. 1981 Mar;41(3):603–609. doi: 10.1128/aem.41.3.603-609.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris D. F., Wolfe N. L., Steen W. C., Baughman G. L. Effect of phenol molecular structure on bacterial transformation rate constants in pond and river samples. Appl Environ Microbiol. 1983 Mar;45(3):1153–1155. doi: 10.1128/aem.45.3.1153-1155.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. E., Schmidt S. Growth of phenol-mineralizing microorganisms in fresh water. Appl Environ Microbiol. 1985 Jan;49(1):11–14. doi: 10.1128/aem.49.1.11-14.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. E., Subba-Rao R. V., Alexander M. Rates of mineralization of trace concentrations of aromatic compounds in lake water and sewage samples. Appl Environ Microbiol. 1982 May;43(5):1133–1138. doi: 10.1128/aem.43.5.1133-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Alexander M. Effects of dissolved organic carbon and second substrates on the biodegradation of organic compounds at low concentrations. Appl Environ Microbiol. 1985 Apr;49(4):822–827. doi: 10.1128/aem.49.4.822-827.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S. K., Simkins S., Alexander M. Models for the kinetics of biodegradation of organic compounds not supporting growth. Appl Environ Microbiol. 1985 Aug;50(2):323–331. doi: 10.1128/aem.50.2.323-331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkins S., Alexander M. Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol. 1984 Jun;47(6):1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain J. C., Van Veld P. A., Monti C. A., Pritchard P. H., Cripe C. R. Comparison of p-Nitrophenol Biodegradation in Field and Laboratory Test Systems. Appl Environ Microbiol. 1984 Nov;48(5):944–950. doi: 10.1128/aem.48.5.944-950.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D., Hijnen W. A. Determination of the concentration of maltose- and starch-like compounds in drinking water by growth measurements with a well-defined strain of a Flavobacterium species. Appl Environ Microbiol. 1985 Apr;49(4):765–771. doi: 10.1128/aem.49.4.765-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D., Visser A., Hijnen W. A. Growth of Aeromonas hydrophila at Low Concentrations of Substrates Added to Tap Water. Appl Environ Microbiol. 1980 Jun;39(6):1198–1204. doi: 10.1128/aem.39.6.1198-1204.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kooij D., Visser A., Oranje J. P. Multiplication of fluorescent pseudomonads at low substrate concentrations in tap water. Antonie Van Leeuwenhoek. 1982;48(3):229–243. doi: 10.1007/BF00400383. [DOI] [PubMed] [Google Scholar]


