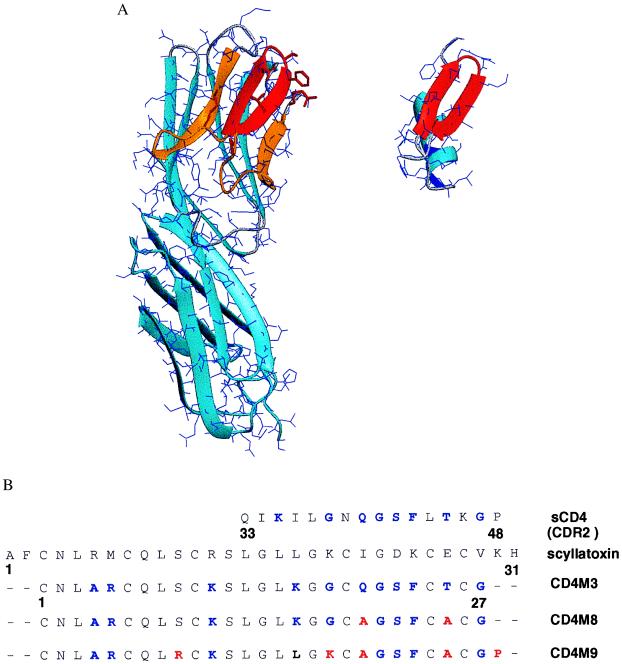
Figure 1.
Structural comparison of CD4 and the scorpion scyllatoxin. (A) Three-dimensional structure of CD4 D1–D2 domains (Left; PDB code 1cdh; ref. 35) and of scyllatoxin (Right; PDB code 1scy; ref. 22). Backbone traces are in light blue ribbons (27). The 25–64 region of CD4, binding to gp120 (7), is in orange, and the 36–47 CDR2-like loop, corresponding to the C’C“ β-hairpin, is in red; the 18–29 β-hairpin of scyllatoxin, structurally similar to the CDR2-like loop, is also in red. CD4 side chains of the CDR2-like loop and R59, at the end of strand D, transferred to scyllatoxin, appear as red sticks. (B) Sequence alignment of the CDR2-like loop of CD4, scyllatoxin, CD4M3, double-mutant CD4M8, and quintuple-mutant CD4M9. Transferred amino acid residues of CD4 and amino acid changes of the engineered scaffolds are in blue; the additional changes that increase affinity for gp120 and antiviral activity are in red.