Abstract
Maize (Zea mays) has a highly branched inflorescence due to the production of different types of axillary meristems. Characterization of the barren inflorescence class of mutants has led to the discovery of genes required for axillary meristem initiation in the inflorescence. Previous studies showed that barren inflorescence2 (bif2) encodes a serine/threonine protein kinase that regulates auxin transport, and barren stalk1 (ba1) encodes a basic helix-loop-helix transcription factor that acts downstream of auxin transport. Here, we characterize Barren inflorescence1 (Bif1), a classical semidominant mutation of maize. Developmental, histological, and genetic analyses show that Bif1 mutants are defective in the initiation of all axillary meristems in the inflorescence. Real time RT–PCR experiments show that both bif2 and ba1 are expressed at lower levels in Bif1 mutants. Double-mutant analyses demonstrate that Bif1 exhibits an epistatic interaction with ba1 and a synergistic interaction with bif2. The dramatic phenotypic enhancement observed in Bif1; bif2 double mutants implies that bif1 plays an overlapping role with bif2 in the initiation of lateral organs during vegetative development. The phenotypic resemblance of Bif1 to bif2 mutants and the reduction of auxin transport in Bif1 mutants suggest that bif1 functions as a regulator of auxin transport in maize.
ORGANOGENESIS in plants is controlled by meristems (Steeves and Sussex 1989). The peripheral zone of the meristem initiates organ primordia while the central zone remains undifferentiated to allow organogenesis to continue indefinitely (McSteen and Hake 1998; Veit 2006). Auxin plays a fundamental role in organogenesis in the peripheral zone of the meristem (Reinhardt et al. 2000, 2003; Vernoux et al. 2000). Plants with mutations in genes required for auxin biosynthesis, transport, or response have defects in organogenesis (Okada et al. 1991; Bennett et al. 1995; Przemeck et al. 1996; Vernoux et al. 2000; Cheng et al. 2006, 2007). These and other studies have shown that auxin is required for leaf initiation during vegetative development and flower initiation during reproductive development (Okada et al. 1991; Reinhardt et al. 2000, 2003; Benkova et al. 2003; Scanlon 2003; Heisler et al. 2005; Cheng and Zhao 2007; Wu and McSteen 2007).
During the vegetative phase of growth, the shoot apical meristem (SAM) at the tip of the developing shoot reiteratively produces phytomers, consisting of node, internode, leaf, and axillary meristem located in the axil of the leaf (Steeves and Sussex 1989; McSteen and Leyser 2005). Axillary meristems can grow out to become a lateral branch, known as a tiller in maize, which reiterates the growth of the main shoot. During the reproductive phase of growth, the shoot apical meristem converts to an inflorescence meristem that produces modified phytomers. In many plants, the leaves are reduced to form bract leaves and the axillary meristems are enlarged to produce the flowers (Steeves and Sussex 1989). In maize, highly branched inflorescences are produced (McSteen et al. 2000; Bommert et al. 2005; Bortiri and Hake 2007). The male inflorescence, the tassel, grows at the apex of the plant and is composed of a main spike with several long branches at the base (Figure 1A). The main spike and branches produce short branches called spikelet pairs. The spikelet is the building block of all grass inflorescences (Clifford 1987; Kellogg 2000). In maize, the spikelet is composed of two leaf-like glumes enclosing two florets. The female inflorescence, the ear, is produced from an axillary meristem located several nodes below the tassel. In both tassel and ear, the florets are enclosed by leaf-like structures called lemma and palea surrounding a pair of petal-like structures, called lodicules, and the reproductive organs, the stamens and carpels. The carpels abort in the tassel and the stamens abort in the ear to produce separate male and female inflorescences (Irish 1996).
Figure 1.—
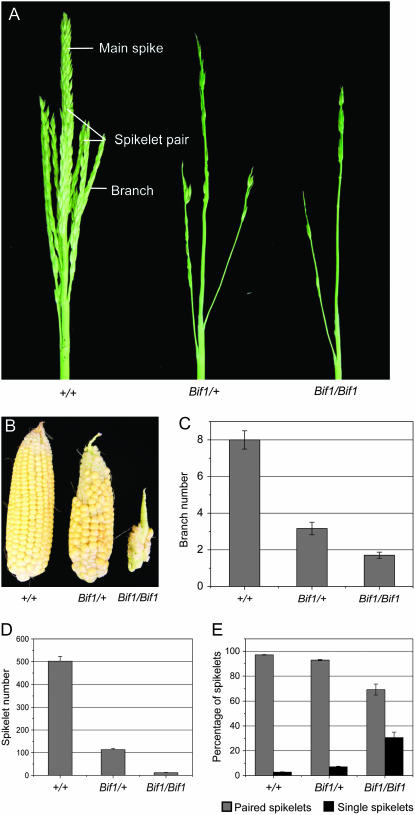
Mature inflorescence phenotype of the Bif1 mutant. (A) Mature tassels of normal, Bif1/+, and Bif1/Bif1 plants. In the normal tassel, long branches are indicated at the base of the main spike. Spikelet pairs cover the branches and the main spike. In the Bif1 mutants, there are reduced numbers of branches and spikelets in the tassel. (B) Mature ears of normal, Bif1/+, and Bif1/Bif1 plants, showing fewer kernels and disorganized rows in Bif1 mutants. (C) Quantification of tassel branch number. (D) Quantification of tassel spikelet number. (E) Percentage of spikelets that occur singly vs. paired. Bars represent mean value and error bars represent standard error of the mean.
To produce this highly branched inflorescence, the inflorescence meristem produces four types of axillary meristems (Table 1), which give rise to the various structures of the mature inflorescence (Cheng et al. 1983; Irish 1997; McSteen et al. 2000; Bommert et al. 2005). The primary axillary meristems have two alternative fates. The first primary axillary meristems that arise are the branch meristems (BMs). BMs are indeterminate and grow out to become long branches at the base of the tassel, which reiterate the growth of the main spike (Table 1). The next primary axillary meristems that arise are the spikelet pair meristems (SPMs). SPMs are determinate and give rise to short branches bearing a pair of spikelets (Table 1). The SPMs produce the secondary axillary meristems called spikelet meristems (SMs), which then produce the tertiary axillary meristems called floral meristems (FMs), which finally produce the floral organs. The fate of the primary axillary meristems as indeterminate (branch) vs. determinate (spikelet pair) is regulated by the ramosa (ra) pathway (Vollbrecht et al. 2005; Bortiri et al. 2006a; McSteen 2006; Satoh-Nagasawa et al. 2006; Kellogg 2007). The ra1 and ra2 genes encode transcription factors that are required to impose determinacy on the SPM (Vollbrecht et al. 2005; Bortiri et al. 2006a). In the ra1 mutant, there are additional long branches in the tassel and the ear (Gernart 1912; Vollbrecht et al. 2005).
TABLE 1.
Axillary meristems in the maize inflorescence
| Order | Meristem | Product | Determinacy |
|---|---|---|---|
| 1° | Branch (BM) | Branches | Indeterminate |
| 1° | Spikelet pair (SPM) | Spikelet pairs | Determinate |
| 2° | Spikelet (SM) | Florets | Determinate |
| 3° | Floral (FM) | Floral organs | Determinate |
The barren inflorescence loci in maize identify genes required for axillary meristem initiation. barren stalk1 (ba1) encodes a basic helix-loop-helix transcription factor required for axillary meristem initiation during both vegetative and reproductive development (Gallavotti et al. 2004). ba1 mutants do not produce tillers, ears, branches, spikelets, and florets (Ritter et al. 2002). barren inflorescence2 (bif2) mutants also have fewer ears and fewer branches, spikelets, florets, and floral organs due to defects in the initiation of axillary meristems in the inflorescence (McSteen and Hake 2001). bif2 mutants also have defects in axillary meristem initiation during vegetative development (McSteen et al. 2007). The bif2 gene encodes a serine/threonine protein kinase co-orthologous to PINOID, which regulates auxin transport in Arabidopsis (Christensen et al. 2000; Benjamins et al. 2001; Lee and Cho 2006; McSteen et al. 2007).
Here, we characterize another barren inflorescence mutation, Barren inflorescence1 (Bif1). Bif1 is a semidominant mutation that confers the phenotype of fewer branches, spikelets, florets, and floral organs in the inflorescence. Although Bif1 is a classical mutation of maize first isolated >30 years ago (Neuffer et al. 1997), the phenotype has not previously been analyzed in detail. Here, we report that the defects in Bif1 mutants are due to defects in the initiation of axillary meristems in the inflorescence. We tested the interaction between Bif1 and bif2 or ba1, using expression and double-mutant analyses. We show that Bif1 is epistatic to ba1 and that ba1 expression is greatly reduced in Bif1 mutants. We show that Bif1 mutants share many phenotypic similarities with bif2 mutants and that bif2 expression is also reduced in Bif1 mutants. The dramatic enhancement of phenotype seen in Bif1; bif2 double-mutant plants indicates that bif1 plays a redundant role with bif2 in the initiation of leaves during vegetative development. Bif1 mutants have reduced levels of auxin transport, implying that the function of bif1 is in the regulation of auxin transport.
MATERIALS AND METHODS
Analysis of the mature inflorescence phenotype of Bif1:
The Bif1-1440 allele was obtained from the Maize Genetics Coop Stock Center (stock no. 827C) and backcrossed eight times into the B73 genetic background. Quantitative analysis was performed on plants grown until maturity (9–10 weeks) in the field during the summer in Rock Springs, Pennsylvania. Data representative of one field season are presented. For analysis of branch and spikelet number, 8–10 plants of each genetic class were analyzed. For floret and floral organ number, 100 spikelets of each genetic class were analyzed.
Double-mutant analyses:
All mutant stocks were backcrossed a minimum of five times to B73 before being used to generate double mutants with Bif1. All double-mutant families were grown in the field during the summer in Rock Springs, Pennsylvania. To reduce environmental effects, all families were planted twice in different field locations and in two separate field seasons. Two to three F2 families of 120 kernels were planted in each location. Data presented here are a representative subset of the data collected during the 2007 field season. Chi-square analysis failed to reject the null hypothesis for the expected number of plants in each genotypic class (supplemental Table S1).
Bif1; ra1:
The ra1-R allele was used to generate Bif1; ra1 double-mutant segregating families (Vollbrecht et al. 2005). At maturity Bif1; ra1 double mutants were scored by tassel and ear phenotype. Inflorescence architecture of at least 10 plants of each genetic class was analyzed. The number of primary and secondary tassel branches and the total number of spikelets were counted. The spikelet number per branch at the base of the tassel was also counted, as well as the number of spikelets in the top two centimeters of the main spike.
Bif1; bif2:
Families segregating Bif1; bif2 double mutants were generated using the bif2-77 allele (McSteen et al. 2007). For genotyping, leaf tissue was collected from 2-week-old plants into 96-well plates and ground using a Tissue Lyzer (QIAGEN, Valencia, CA). DNA was extracted according to a protocol modified for 96-well plate format from Chen and Dellaporta (1994) with the phenol chloroform extraction step omitted. PCR was carried out to genotype the plants for the bif2-77 mutation, using primers bif2-57 (5′ CAG CCT GCC GCG CTG CTC CAGC 3′) and bif2-250 (5′ CGG CGC AGC AGC CTG AAG TCC 3′), which are designed to cross the site of the insertion in this bif2 allele (McSteen et al. 2007). A second set of PCR reactions, using primer bif2-57 with a primer located in the insertion (bif2-77, 5′ CAG TGG CGG GCC TAG AAA TTT G 3′), was used to confirm this result. Bif1/Bif1; bif2/bif2 plants were easily identified as plants genotyped as homozygous for the bif2 mutation but that also had extremely short stature and a severe tassel phenotype. Initial phenotype analysis revealed an excess of plants with a Bif1/Bif1 homozygous phenotype. This excess was attributed to Bif1/+; bif2/bif2 double mutants that resembled severe Bif1 homozygotes in phenotype but were genotyped as homozygous for the bif2 mutation. Further confirmation of this result was obtained by crossing Bif1/+; bif2/+ plants to +/+; bif2/+ plants and determining that one-eighth of the progeny resembled Bif1 homozygotes.
At maturity, plant height was measured on every plant from the ground to the tip of the tassel. To count leaf number, every fifth leaf of each plant was clipped with pinking shears, beginning at 3 weeks after emergence and at regular intervals throughout the field season. This enabled us to obtain an accurate measure of total leaf number at the end of the field season because if we had counted only at the end of the field season, we would have missed the leaves that had senesced. Ten plants of each genetic class were used for analysis of tassel branch number and spikelet number.
Bif1; ba1:
The ba1-ref allele was used to generate Bif1; ba1 double-mutant segregating families (Gallavotti et al. 2004). Tissue was collected and DNA extracted as described for the Bif1; bif2 plants. Plants were genotyped using primer ba04 (5′ TGG CAT TGC ATG GAA GCG TGT ATG AGC 3′) located in the ba1 promoter and primer ba05 (5′ TCC TAG ACA TGC ATA TCT GAA CCA GAG CT 3′) located in the helitron in the ba1-ref allele, which amplified a product in ba1 heterozygous and homozygous plants. A second PCR reaction with primers ba04 and ba07 (5′ GCT AAG CTA CTG TAA GCG GGA TGG ACA 3′) amplified a product in wild-type and heterozygous plants. Bif1/Bif1; ba1/ba1 double mutants were classified as plants genotyped as homozygous for ba1, but with a smooth, thin tassel rachis similar to Bif1 homozygotes. Bif1/+; ba1/ba1 double mutants were classified as plants genotyped as homozygous for ba1, which looked like ba1 but with a slightly smoother tassel rachis.
Statistical analysis:
The computer program Minitab v.15 (Minitab, State College, PA) was used to perform all statistical analysis. Data sets were compared with two-sample two-tailed t-tests. Data presented in bar charts are the mean value of the data, and all error bars show standard error of the mean.
Scanning electron microscopy, RNA in situ hybridization, and histology:
Tassels were obtained from families segregating for Bif1 grown in the greenhouse for 5 weeks. The tassels were dissected and fixed on ice overnight in 3.7% formalin, 50% ethanol, 10% acetic acid (FAA) and then dehydrated through an ethanol series. Ears were obtained from Bif1 plants grown in the field for 8 weeks. Ears were dissected and fixed on ice overnight in 4% formaldehyde in phosphate-buffered saline. For scanning electron microscopy (SEM), meristems were critical-point dried (BAL-TEC CPD 030; Techno Trade, Manchester, NH) and then mounted onto carbon stubs. The samples were sputter coated with a 0.7-Å layer of gold palladium (BAL-TEC SCD 050, Techno Trade) and viewed by SEM (JSM 5400; JEOL, Peabody, MA), using a 10-kV accelerating voltage. For sectioning, samples were embedded in paraffin wax (Paraplast Plus; McCormick Scientific, St. Louis). Sections 8 μm thick were cut using a Finnesse paraffin microtome (Thermo Fisher, Waltham, MA) and mounted onto coated slides (Probe-On Plus; Fisher Scientific, Waltham, MA). For RNA in situ hybridization, the slides were probed with a DIG-labeled RNA antisense probe of kn1 according to Jackson et al. (1994). For histology, the slides were dewaxed using histoclear (National Diagnostics, Atlanta), hydrated through an ethanol series, stained in 0.05% Toluidine Blue O (TBO) for 30 sec, dehydrated, and mounted with a coverslip using Histomount (Thermo-Shandon, Pittsburgh). All slides were viewed under bright field with an Eclipse 80i upright microscope (Nikon, Melville, NY) and photographed with a DXM1200F digital camera (Nikon).
Expression analysis:
Total RNA was isolated from 5- to 6-week-old tassels (5–7 mm) and 8-week-old ears (20–22 mm) from Bif1 homozygotes and normal siblings, using the Nucleospin RNA plant kit following the manufacturer's protocol (Macherey-Nagel, Durel, Germany). One tassel or ear (∼8–12 mg fresh weight) was used per RNA extraction, with three biological replicates of each sample type. A total of 200 ng of RNA from each sample were DNase treated, using the DNase I kit (Ambion, Austin, TX) to remove genomic DNA contamination. Reverse transcription was carried out using the ABI High-Capacity RT kit (Applied Biosystems, Foster City, CA), with incubation at 25° for 10 min and then 37° for 2 hr. Real-time RT–PCR primers and 5′ FAM- (Carboxyfluorescein) and 3′ BHQ1- (Black Hole Quencher) labeled Taqman probes (Biosearch Technologies, Novato, CA) were designed, using Primer Express version 2.0 software (Applied Biosystems). Five microliters of cDNA were used as template for real-time RT–PCR reactions using TaqMan 2X Universal mix (Applied Biosystems), except that Betaine (Sigma, St Louis) was added to a final concentration of 0.5 m in the bif2 reactions. RT–PCR reactions were carried out in 96-well plates using an ABI 7300 real-time PCR machine (Applied Biosytems). For detection of bif2 expression, the Taqman probe was (FAM-5′ CTC CGC CAC CGC ATG CCC 3′-BHQ) and the RT–PCR primers were bif2F (5′ CTG CGT CGT CAC GGA GTT C 3′) and bif2R (5′ TGC CCA TCA TGT GCA GGT ACT 3′). For detection of ba1 expression, the Taqman probe was (FAM-5′ ACG CGG CTT CCC CAT CAT CCA 3′-BHQ) and the RT–PCR primers were ba1F (5′ TGG ATC CAT ATC ACT ACC AAA CCA 3′) and ba1R (5′ ACC GGG TGC TGG AGG TAA G 3′). The control for normalization was ubiquitin: the Taqman probe was (5′ FAM-AAA TCC ACC CGT CGG CAC CTC C 3′-BHQ) and RT–PCR primers were ubqF (5′ CTC TTT CCC CAA CCT CGT GTT 3′) and ubqR (5′ ACG AGC GGC GTA CCT TGA 3′). Three technical replicates of each real-time PCR reaction were performed on three biological replicates for each experiment and the entire experiment was repeated twice. Normalized relative expression levels were determined using the comparative threshold method (Livak and Schmittgen 2001).
Auxin transport assays:
Auxin transport assays were performed using a method modified from Okada et al. (1991) and McSteen et al. (2007). Immature ear inflorescences were dissected from plants grown in the field for 8 weeks. The immature ears ranged from 2 to 3 cm in size. At this stage of development, the inflorescence meristem was still initiating SPMs at the tip and floral organs were being produced at the base. Two centimeters of the tip of the ear was placed in either orientation into 2-ml tubes containing 100 μl 1.5 μm 3-[5(n)-3H] indole acetic acid (specific activity 25 Ci/mmol; GE Healthcare, Piscataway, NJ) in 0.5× Murashige and Skoog medium (Sigma, St. Louis). Some tubes also contained 20 μm N-1-naphthylphthalamic acid (Chemservice, West Chester, PA). After 24 hr incubation in the dark, the immature ear pieces were blotted and 5 mm from the end that was not immersed in solution was placed in scintillation fluid (Ready safe; Beckman Coulter, Fullerton, CA) and counted in a liquid scintillation counter (LSC6000, Beckman Coulter). For the initial experiment on normal ears, three ears were used for each treatment and the experiment was repeated three times. For the experiment on Bif1 mutants, three ears from each genotypic class were used and the experiment was repeated four times. Data that are representative of one experiment are presented.
RESULTS
The Bif1 mutation was recovered from an EMS mutagenesis experiment (Neuffer and Sheridan 1977) and was mapped to chromosome 8 using genetic and cytogenetic tools (http://www.maizegdb.org). Using SSR markers, we fine mapped Bif1 to between idp98 and umc1360 in bin 8.02. Bif1 is a semidominant mutation with the homozygote having a more severe phenotype than the heterozygote. The Bif1 mutation confers the phenotype of fewer branches and spikelets in the tassel and fewer kernels in the ear but the phenotype had not previously been analyzed in detail (Coe et al. 1988; Sheridan 1988; Veit et al. 1993; Neuffer et al. 1997; McSteen et al. 2000).
Bif1 mutants produce fewer branches and spikelets:
Bif1 mutant tassels had a sparse appearance with fewer branches and spikelets compared to normal siblings (Figure 1A). The tassels of plants that were homozygous for Bif1 were more strongly affected than heterozygotes (Figure 1A). Plants heterozygous for Bif1 produced ears with irregular rowing due to the reduced number of kernels and the tip was barren (Figure 1B). Plants homozygous for Bif1 produced ears with very few kernels, such that bare rachis was visible (Figure 1B).
Quantitative analysis of the mature Bif1 tassel showed that Bif1 mutants fail to produce the full complement of tassel branches (Figure 1C). Analysis of spikelet number showed a statistically significant reduction in spikelet number in plants heterozygous and homozygous for Bif1 (Figure 1D). In plants homozygous for Bif1, spikelets that formed were sometimes produced singly instead of in pairs (Figure 1E). The reduced number of branches and spikelets produced suggests that the initiation or maintenance of primary axillary meristems, the BM and SPM, is defective in Bif1 inflorescences.
Bif1 mutants fail to initiate SPMs:
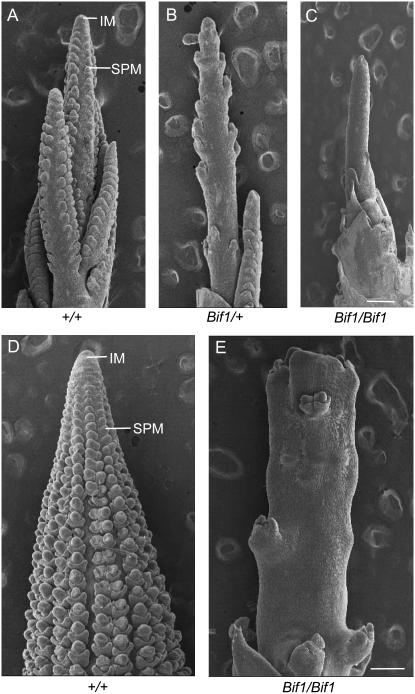
The phenotype of the mature Bif1 inflorescence suggested that there were defects in the early stages of inflorescence development. To test this, SEM was used to visualize the developing inflorescence. By 5 weeks of growth, normal inflorescences had initiated several lateral branches, with SPMs visible as regular bumps on the flanks of both the main spike and the branches (Figure 2A). At the same stage of development, plants heterozygous for Bif1 had a reduced number of SPMs (Figure 2B). Plants homozygous for Bif1 had a more severe phenotype with very few or, in some cases, no SPMs (Figure 2C). The barren surface of the rachis was very slightly ridged (Figure 2C). The Bif1 homozygous ear had a similar phenotype as the tassel with few SPMs initiated (Figure 2E). Unlike the tassel, the ear inflorescence meristem was fasciated (Figure 2E).
Figure 2.—
Scanning electron microscopy (SEM) images of developing Bif1 inflorescences. (A) Normal tassel, showing files of developing spikelet pair meristems (SPMs) on the flanks of the inflorescence meristem (IM). (B) Bif1/+ tassel, with reduced numbers of SPMs. (C) Bif1/Bif1 tassel with few SPMs. (D) Normal ear, showing organized rows of SPMs. (E) Bif1/Bif1 ear, with a fasciated inflorescence meristem and few SPMs. Bar, 100 μm.
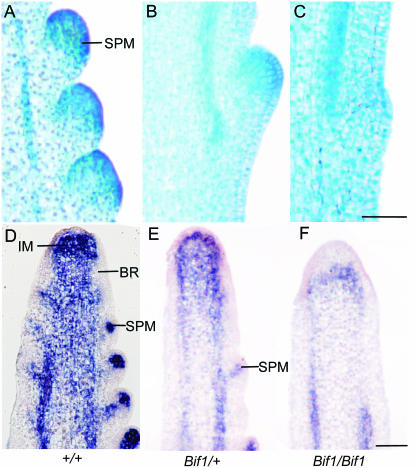
To determine if there was any histological evidence of SPM formation, we used TBO to stain sections of developing Bif1 inflorescences. As meristematic cells have smaller vacuoles than differentiated cells, SPMs stain more intensely with TBO than surrounding tissue. In normal inflorescences, developing SPMs were visible as regular groups of densely staining cells on the flanks of the inflorescence (Figure 3A). In Bif/+ plants, SPMs visible on the flanks of the inflorescence looked similar to normal except there were fewer of them (Figure 3B). Bif1 homozygotes mostly did not produce SPMs (Figure 3C). Instead, the surface of barren regions of the inflorescence occasionally had very slightly raised protrusions that were less intensely stained than SPMs, indicating that the slight ridges visible by SEM consist of differentiated tissue (Figure 3C).
Figure 3.—
Histology and RNA in situ hybridization with kn1 in developing Bif1 tassels. (A–C) Longitudinal sections of 5-week-old tassels stained with TBO, with SPMs visible as areas of intense staining. (A) Three developing SPMs on the flanks of the inflorescence meristem in a normal tassel. (B) Bif1/+, showing a single SPM in the same area as there are three SPMs in normal. (C) Bif1/Bif1 with a slight protrusion on the surface of the rachis but no evidence of developing SPM. (D–F) RNA in situ hybridization with kn1. (D) Meristematic cells and vasculature are indicated by kn1 expression in normal tassels. The absence of kn1 on the flanks of the inflorescence meristem (IM) indicates the formation of the suppressed bract primordia (BR) that subtend SPMs. (E) Bif1/+ inflorescences have fewer areas of kn1 expression on the flanks of the inflorescence. (F) Bif1/Bif1 inflorescence with kn1 expression only in the inflorescence meristem and in the vasculature. Bar, 100 μm.
To determine if there was any molecular evidence of SPM formation, RNA in situ hybridization with kn1 was used as a marker to identify meristematic tissues. kn1 is expressed in meristems, where it is required for meristem maintenance, and is not expressed as organ primordia differentiate (Jackson et al. 1994; Kerstetter et al. 1997; Vollbrecht et al. 2000). In normal plants, kn1 expression was clearly visible in the inflorescence meristem and in the vasculature and stem (Figure 3D). kn1 was not expressed on the flanks of the inflorescence meristem, as bract primordia (whose subsequent growth is suppressed) initiate (labeled BR in Figure 3D). However, kn1 was strongly expressed in SPMs that form in the axils of bract primordia (Figure 3D). In Bif1 mutants, kn1 was expressed as normal in the inflorescence meristem, vasculature, and stem (Figure 3, E and F). In plants heterozygous for Bif1, areas with no kn1 expression were interspersed with areas of kn1 expression as expected for the few SPMs that initiate (Figure 3E). In plants homozygous for Bif1, there was usually no evidence of SPM formation on the flanks of the inflorescence meristem (Figure 3F). As downregulation of kn1 was visible on the flanks of the Bif1 inflorescence meristem (Figure 3, E and F), this indicates that bract primordia are set aside in Bif1 mutants and that the occasional small ridges visible in Bif1 mutants may be suppressed bract primordia. However, the in situs with kn1 clearly show that SPMs do not form in the axils of the bract primordia in plants homozygous for Bif1.
Bif1 mutants have defects in SM initiation rather than in SPM determinacy:
In normal plants, on the main spike and lateral branches, the SPMs produce a pair of SMs. Bif1 mutants have a reduced number of spikelets, in large part due to the reduced numbers of SPMs. When SPMs initiate in Bif1 mutants they often produce single instead of paired spikelets (Figure 1E). This indicates that Bif1 mutants have defects either in SM initiation or in SPM determinacy. To distinguish between these two possibilities, we constructed double mutants between Bif1 and ramosa1 (ra1). ra1 encodes an EPF zinc-finger transcription factor that confers determinacy on the SPM (Vollbrecht et al. 2005). In ra1 mutants, SPMs lack determinacy and grow out to become long indeterminate branches instead of producing determinate spikelet pairs (Gernart 1912; Vollbrecht et al. 2005). As a result, ra1 mutants produce additional long branches in both the tassel and the ear (Figure 4, A and B).
Figure 4.—
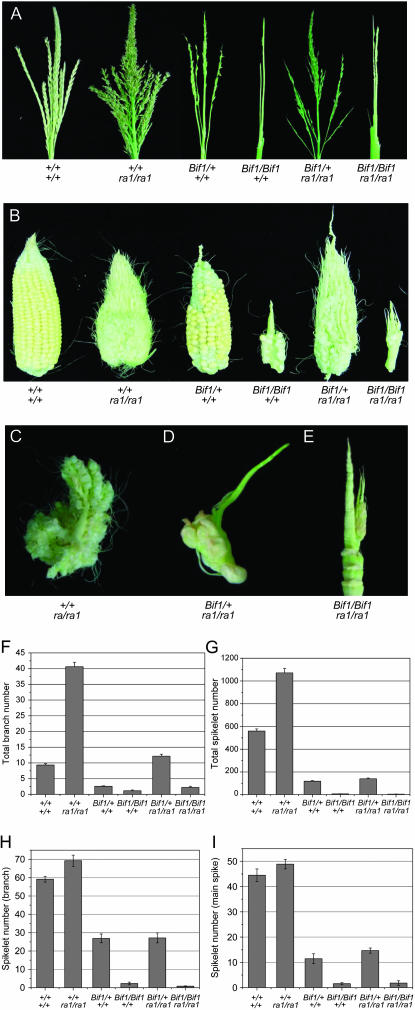
Analysis of Bif1; ra1 double mutants. (A) Mature tassel phenotype showing all genetic classes from a segregating Bif1; ra1 family. (B) Ear phenotype of a segregating Bif1; ra1 family. (C–E) Higher-magnification images showing individual branches from ears. (C) Branch from a ra1/ra1 ear. (D) Branch from a Bif1/+; ra1/ra1 ear. (E) Bif1/Bif1; ra1/ra1 ear. (F–I) Quantitative analysis of Bif1; ra1 double mutants. For all charts, bars represent mean value of the data set, and error bars represent standard error of the mean. (F) Average number of branches per tassel. (G) Average number of spikelets per tassel. (H) Average number of spikelets per branch, measured on a branch at the base of the tassel. (I) Average number of spikelets in the top 2 cm of the tassel main spike.
We found that even in the ra1 mutant background, Bif1 homozygotes were unable to make additional long branches in the tassel (Figure 4A). The Bif1/Bif1; ra1/ra1 double mutant had a barren tassel phenotype similar to Bif1 (Figure 4A). Quantitative analysis showed that total branch number and spikelet number were not statistically different between Bif1/Bif1; ra1/ra1 and Bif1/Bif1 (branch number, P-value = 0.054; spikelet number, P-value = 0.192; Figure 4, F and G). Therefore, when no SPMs were produced in Bif1 homozygotes, ra1 could not act on them. On the other hand, in the Bif1/Bif1; ra1/ra1 ear, several branches grew out from the rachis (Figure 4B, close-up shown in Figure 4E). It appeared that when SPMs initiated in the Bif1/Bif1; ra1/ra1 ear, they converted to branches due to the absence of ra1 (Figure 4, B and E). Hence, the Bif1 mutant does not have defects in SPM determinacy once SPMs have initiated.
Further insight was obtained by characterizing Bif1/+; ra1/ra1 double mutants. The tassel of Bif1/+; ra1/ra1 double mutants had more long branches and spikelets than Bif1/+ (Figure 4A). Quantitative analysis showed that there was a significant increase in branch number in Bif1/+; ra1/ra1 compared to Bif1/+ (P-value <0.001, Figure 4F). This suggests that when SPMs initiated, they grew out to become lateral branches. However, the branches on Bif1/+; ra1/ra1 were more barren than typical ra1 branches and did not have a statistically different number of spikelets compared to Bif1/+ plants (P-value = 0.94; Figure 4, G–I). Similarly, the Bif1/+; ra1/ra1 ear was more highly branched than the Bif1/Bif1; ra1/ra1 ear but few spikelets were produced on the branches (Figure 4B, close-up of an individual branch shown in Figure 4D). These results indicate that Bif1 mutants have defects in the initiation of secondary axillary meristems, SMs, rather than defects in the determinacy of primary axillary meristems, SPMs.
Spikelet and floral meristems are defective in Bif1 mutants:
Dissection of the few spikelets produced in Bif1 mutant plants indicated that Bif1 spikelets had fewer florets than normal and the florets had fewer floral organs. To quantify these defects, 100 spikelets were dissected from both Bif1 heterozygous and homozygous plants and the number of florets and floral organs was determined relative to normal sibs.
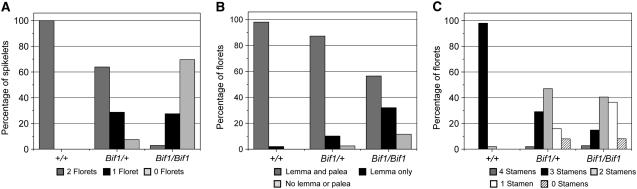
In normal plants, each spikelet bears a pair of florets (Figure 5A). In Bif1 heterozygous plants, only 64% of spikelets produced two florets (Figure 5A), while in Bif1 homozygotes only 3% of spikelets produced two florets (Figure 5A). These results indicate that in Bif1 mutants, SMs are defective as they are unable to initiate the normal number of tertiary axillary meristems, FMs.
Figure 5.—
Quantification of floret and floral organ numbers in Bif1 mutants. (A) Percentage of spikelets containing two, one, or zero florets per spikelet. (B) Quantification of lemma and palea number per floret. (C) Percentage of florets containing the indicated number of stamens per floret.
Normal tassels produce florets that contain a lemma, a palea, two lodicules, and three stamens. Quantitative analysis indicated that in Bif1 mutants, the florets had fewer floral organs than normal with the homozygote being more severely affected than the heterozygote (Figure 5, B and C). The number of lemmas and paleae was reduced in Bif1 mutants with 87% of spikelets from Bif1 heterozygotes and 56% of spikelets from Bif1 homozygotes producing both organs (Figure 5B). Lodicules were not counted as their small size and transparency made them difficult to count with accuracy under a dissecting microscope. Stamen number was reduced in Bif1 mutants with only 29% of Bif1/+ florets and 12% of Bif1/Bif1 florets containing the normal three stamens (Figure 5C). Interestingly, a small percentage of Bif1 mutant florets contained four stamens, indicating that floral organ number could be increased as well as decreased. The failure to initiate the normal number of floral organs indicates that FMs are also defective in Bif1 mutants.
Expression studies show that bif2 and ba1 are expressed at a lower level in Bif1 mutants:
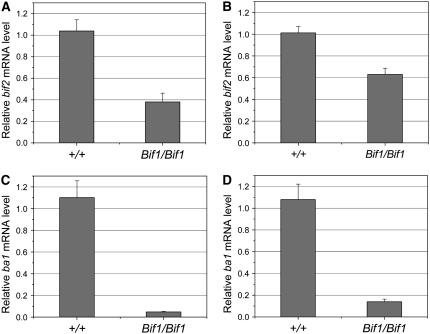
Like Bif1, bif2 and ba1 mutants are also defective in the initiation of all types of axillary meristems in the inflorescence (McSteen and Hake 2001; Ritter et al. 2002). To determine whether the Bif1 mutation affected the expression of bif2 or ba1, real-time RT–PCR experiments were performed. bif2 and ba1 are both expressed in tassels and ears in normal plants (Gallavotti et al. 2004; McSteen et al. 2007). bif2 is expressed in axillary meristems, lateral organs, and vasculature while ba1 has a more restricted expression pattern during axillary meristem initiation (Gallavotti et al. 2004; McSteen et al. 2007). RNA was isolated from immature tassels and ears of plants homozygous for Bif1 and from normal siblings. Real-time RT–PCR experiments indicated that both bif2 and ba1 RNA levels were reduced in tassels and ears of plants homozygous for Bif1 (Figure 6, A–D). bif2 levels were reduced to 36–62% of normal levels in tassel and ears, respectively (Figure 6, A and B), some of which could be explained by the reduction in the number of BMs, SPMs, SMs, FMs, and floral organs in Bif1 mutants. On the other hand, ba1 levels were dramatically reduced to 4–13% of normal levels (Figure 6, C and D). Considering that ba1 is expressed in a very restricted pattern as axillary meristems initiate, these results provide further support that the Bif1 mutation affects early stages of axillary meristem initiation.
Figure 6.—
Real-time RT–PCR analysis of the expression of bif2 and ba1 in Bif1 mutants. (A) Expression level of bif2 in the immature tassel of Bif1 mutants relative to normal siblings. (B) Expression level of bif2 in the immature ears of Bif1 mutants relative to normal siblings. (C) Expression level of ba1 in the immature tassel of Bif1 mutants relative to normal siblings. (D) Expression level of ba1 in the immature ears of Bif1 mutants relative to normal siblings. Mean plus or minus SE is shown for one representative experiment using three biological and three technical replicates for each sample.
Double-mutant analysis indicates that bif1 and bif2 play a role in vegetative development:
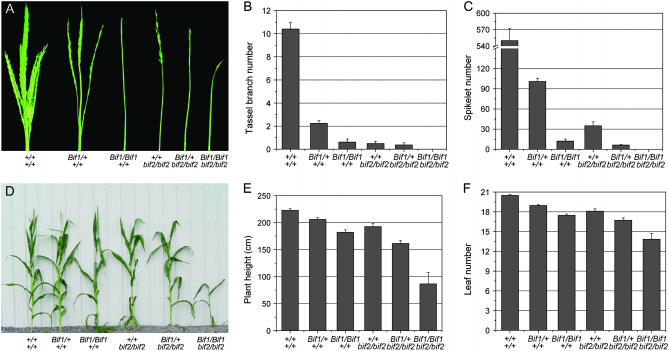
bif2 mutants have a phenotype very similar to that of Bif1 homozygotes, with very few tassel branches and spikelets (McSteen and Hake 2001; McSteen et al. 2007). To determine the genetic interaction between Bif1 and bif2, double-mutant lines were constructed. Bif1; bif2 double-mutant plants had very dramatic effects on both vegetative and inflorescence development.
Inflorescence phenotype:
The inflorescence phenotype of Bif1; bif2 double mutants was more severe than that of either single mutant with no branches or spikelets (Figure 7, A–C). Quantitative analysis showed that the absence of spikelets in Bif1/Bif1; bif2/bif2 double mutants was a statistically significant reduction in spikelet number compared to Bif1/Bif1 (P-value = 0.004) or bif2 single mutants (P-value = 0.001, Figure 7C). Furthermore, genetic and molecular analyses indicated that plants that were heterozygous for Bif1 and homozygous for bif2 resembled Bif1 homozygotes (Figure 7, A–C). These results suggest that bif1 and bif2 play redundant roles in branch and spikelet initiation in the inflorescence.
Figure 7.—
Analysis of Bif1; bif2 double mutants. (A) Mature tassel phenotype of a Bif1; bif2 segregating family. (B and C) Quantification of tassel characteristics in a Bif1; bif2 segregating family. (B) Average tassel branch number. (C) Average spikelet number per tassel. (D) Vegetative phenotype of a Bif1; bif2 family showing reduced plant height in Bif1; bif2 double mutants. (E and F) Quantification of vegetative phenotypes. (E) Average plant height in centimeters. (F) Average leaf number.
Vegetative phenotype:
Bif1; bif2 double-mutant plants were less than half the height of normal plants (Figure 7, D and E). To determine if the reduction in plant height was due to a difference in the number of phytomers produced, the number of leaves were counted (Figure 7F). Both Bif1 and bif2 (McSteen et al. 2007) have a minor effect on leaf number on their own, with a small but statistically significant reduction in the number of leaves compared to normal siblings (P-value = 0.001, Figure 7F). However, the Bif1/Bif1; bif2/bif2 double mutant had a nonadditive effect with a large and significant reduction in leaf number compared to either Bif1/Bif1 (P-value <0.001) or bif2/bif2 (P-value = 0.001) single mutants. The dramatic effect on leaf number in the Bif1; bif2 double mutant implies that bif1 and bif2 also play redundant roles in the production of leaves by the vegetative shoot apical meristem.
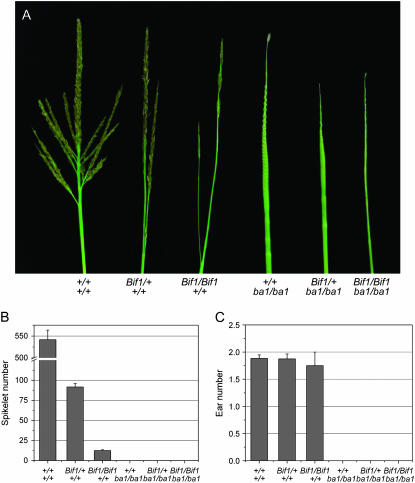
Double-mutant analysis indicates that Bif1 is epistatic to ba1 in the tassel:
The barren stalk1 (ba1) mutant is deficient in both vegetative and inflorescence axillary meristem initiation and as a result lacks tillers and ears, as well as branches and spikelets in the tassel (Ritter et al. 2002; Gallavotti et al. 2004). Although epistasis is challenging to determine when mutants have a similar phenotype, Bif1 mutants can be distinguished from ba1 mutants by the appearance of the inflorescence stem (rachis). Bif1 mutants have a smooth thin rachis, while ba1 mutants have a thick rachis with very regular pronounced protrusions due to the production of larger than normal suppressed bract primordia (Ritter et al. 2002). Bif1/+; ba1/ba1 double mutants resembled ba1 single mutants; however, the surface of the rachis was slightly smoother than usually observed in ba1 tassels (Figure 8A). Bif1/Bif1; ba1/ba1 double mutants resembled Bif1 homozygotes with a smooth thin tassel rachis. As the Bif1/Bif1; ba1/ba1 double mutant abolished the regular protrusions normally seen in ba1 mutants, this indicates that Bif1 is epistatic to ba1 in the tassel. The inflorescence phenotype of the double mutant was not enhanced with respect to spikelet number, which was not unexpected as ba1 mutants typically do not produce any spikelets (Figure 8B). Similarly, the double mutant did not produce any ears (Figure 8C). Moreover, this analysis also showed that the Bif1 mutants alone did not have any defects in the production of ears (Figure 8C). Unlike the interaction between Bif1 and bif2, there was no enhancement of the vegetative defects of Bif1 by ba1 (data not shown), indicating that ba1 does not play a redundant role in leaf initiation during vegetative development.
Figure 8.—
Analysis of Bif1; ba1 double mutants. (A) Mature tassel phenotype of Bif1; ba1 family. (B) Average number of spikelets per tassel. (C) Average number of ears per plant.
Bif1 mutants have a reduced level of auxin transport:
As Bif1 mutants had such a dramatic interaction with bif2, which plays a role in auxin transport, we tested whether Bif1 mutants also have defects in auxin transport. bif2 mutants have a reduced level of auxin transport in the mature inflorescence stem of the tassel (McSteen et al. 2007). Preliminary experiments showed that Bif1 mutants similarly had reduced transport in the mature tassel inflorescence stem (data not shown). However, both Bif1 and bif2 mutants have reduced vasculature in the mature inflorescence stem (McSteen et al. 2007 and data not shown). Bif1 mutants also have a reduction in vasculature in the immature tassel inflorescence early in development (supplemental Figure S1, A–C). However, Bif1 mutants do not have significant reduction in vasculature in the developing ear inflorescence (supplemental Figure S1, D–F). Therefore, to determine if Bif1 mutants had defects in auxin transport early in development, we developed a protocol to measure auxin transport within the ear inflorescence.
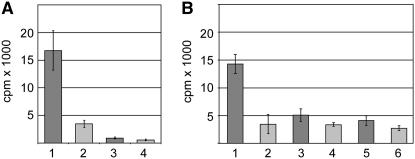
Immature ear inflorescences up to 2 cm in length were incubated overnight in either orientation in a solution of 1.5 μm 3H-labeled IAA. Wild-type ears showed an appreciable level of basipetal transport that was inhibited by co-incubation with 20 μm N-1-naphthylphthalamic acid (NPA), a frequently used auxin transport inhibitor (Figure 9A, lanes 1 and 2). However, acropetal transport was very low at this point in development (Figure 9A, lanes 3 and 4).
Figure 9.—
Measurement of auxin transport in normal and Bif1 inflorescences. Dark shading, without NPA; light shading, with NPA. (A) Measurement of basipetal (lanes 1 and 2) and acropetal (lanes 3 and 4) transport in normal ears. (B) Measurement of basipetal transport in immature ears of a family segregating for Bif1. Lanes 1 and 2, normal siblings; lanes 3 and 4, Bif1/+; lanes 5 and 6, Bif1/Bif1.
To test whether Bif1 ear inflorescences had a reduced level of auxin transport, basipetal transport was measured in plants that were heterozygous or homozygous for Bif1 compared to normal siblings (Figure 9B). Plants that were heterozygous for Bif1 had approximately one-third the level of transport as normal siblings (Figure 9B, lane 3), and these levels were further reduced by co-incubation with 20 μm NPA (Figure 9B, lane 4). Homozygous Bif1 ears had an ever further reduction in active auxin transport (Figure 9B, lane 5), which was not significantly different from that of normal siblings treated with NPA (P-value = 0.75). Therefore, Bif1 mutants have a reduced level of auxin transport, indicating that the primary defect in Bif1 mutants may be in the regulation of auxin transport.
DISCUSSION
We have identified a new player in the pathway for axillary meristem initiation during maize inflorescence development. Bif1 mutants have a very similar phenotype to bif2 mutants with defects in the initiation of all axillary meristems in the inflorescence. The synergistic interaction of Bif1 with bif2 indicates that bif1 acts redundantly with bif2 during both vegetative and inflorescence development. We propose that the defects in Bif1 mutants are caused by a reduction in auxin transport and that the function of bif1 is to regulate auxin transport.
bif1 plays a role in axillary meristem initiation:
Plants that are homozygous for Bif1 have a very similar phenotype to bif2 mutants (McSteen and Hake 2001). Similarities with bif2 mutants include a reduction in the number of branches, spikelets, florets, and floral organs in the tassel and a reduction in kernel number in the ear. Moreover, Bif1 mutants produce single instead of paired spikelets, which is also characteristic of bif2 mutants. The tassel and ear rachis is smooth with occasional irregular ridges, similar to bif2. In addition, the apical ear inflorescence meristem can be fasciated, similar to bif2.
Characterization of the developing inflorescence by SEM analysis, histology, and kn1 expression shows that there is a specific defect in axillary meristem initiation in Bif1 mutants. We propose that bif1 plays a role in axillary meristem initiation in the inflorescence. All axillary meristems in the inflorescence—BM, SPM, SM, and FM—are affected in the mutants. However, unlike bif2, Bif1 mutants do not have defects in the initiation of the axillary meristem that gives rise to the ear shoot. Ears are produced in Bif1 mutants as normal and there is no enhancement of the ear number defects in Bif1; bif2 double mutants (data not shown). Moreover, unlike bif2, double-mutant analysis with teosinte branched1 (tb1) (Doebley et al. 1997; Hubbard et al. 2002; McSteen et al. 2007) shows that the Bif1 mutation does not have a major effect on vegetative axillary meristem (tiller) production (data not shown). Therefore, one of the few differences between Bif1 and bif2 mutations is the extent of their effect on tiller and ear production.
During vegetative development, Bif1 mutants have a small but significant reduction in the number of leaves, resulting in a concomitant reduction in plant height. bif2 mutants also have a minor effect on the initiation of leaves during vegetative development (McSteen et al. 2007). The dramatic effect of the Bif1; bif2 double mutant on vegetative development indicates that bif1 and bif2 play redundant roles in the production of leaves by the vegetative apical meristem. Therefore, our analysis shows that in addition to the role of bif1 and bif2 in initiation of axillary meristems during inflorescence development, bif1 and bif2 also play overlapping roles in the production of lateral organs during vegetative development.
Role of bif1 in auxin transport:
Gradients of auxin are required for polar growth in plants (Benkova et al. 2003; Heisler et al. 2005). In pinformed1 (pin1) and pinoid (pid) mutants in Arabidopsis, a reduction in auxin transport abolishes the initiation of axillary meristems, leading to a “pin” inflorescence phenotype analogous to the barren inflorescence phenotype in maize (Okada et al. 1991; Bennett et al. 1995; Galweiler et al. 1998; Reinhardt et al. 2003). Double mutants in members of the YUCCA gene family, required for auxin biosynthesis, also cause a pin inflorescence phenotype (Cheng et al. 2006). However, either loss or gain of function of the transcription factor MONOPTEROS leads to a pin inflorescence phenotype, illustrating that loss or gain of auxin signaling abolishes axillary meristem initiation (Przemeck et al. 1996; Hardtke et al. 2004). Therefore, defects in auxin biosynthesis, transport, or response lead to a failure to initiate axillary meristems in the inflorescence in Arabidopsis (Cheng and Zhao 2007).
We propose that bif1 acts together with bif2 in the control of auxin transport in the maize inflorescence. Many of the phenotypes seen in Bif1 and bif2 mutants are also seen in plants treated with auxin transport inhibitors (Wu and McSteen 2007). For example, the failure to initiate axillary meristems in the inflorescence, single spikelets, reduced vasculature, and fewer leaves are also seen in plants that have been treated with polar auxin transport inhibitors (Scanlon 2003; Wu and McSteen 2007). Therefore, we tested the levels of auxin transport in the inflorescence of Bif1 mutants and found auxin transport to be reduced, implying that bif1 plays a role in auxin transport.
The Bif1 mutation is semidominant so it could be either a dominant loss-of-function (e.g., antimorph or hypomorph) or a dominant gain-of-function (e.g., hypermorph or neomorph) mutation. It was not possible to use dosage analysis to determine whether Bif1 is a loss- or gain-of-function mutation as Bif1 is not uncovered by the known translocation lines on chromosome 8. However, as the Bif1 mutation causes a reduction of auxin transport, we can conclude that the bif1 gene is either a positive or a negative regulator of auxin transport.
Genetic interaction between Bif1 and other barren inflorescence mutations:
To determine the genetic interaction between Bif1 and previously known barren inflorescence mutations we performed double-mutant and expression analyses. We infer that bif1 acts upstream of ba1 as the Bif1; ba1 double mutant resembled Bif1 in the tassel. In addition, the levels of ba1 expression were dramatically reduced in Bif1 mutants. Further support for this hypothesis is provided by the proposal that ba1 acts downstream of auxin transport (Wu and McSteen 2007). The ba1 mutant produces bracts in a very regular pattern, indicating that phyllotaxis is not disrupted in the mutant and that auxin transport is normal (Ritter et al. 2002). Furthermore, ba1 is not expressed after treatment with auxin transport inhibitors, indicating that ba1 expression depends on auxin transport (Wu and McSteen 2007). We propose that ba1, being a transcription factor, is required for the response to the auxin signal for axillary meristem initiation. We propose that bif1 acts upstream of auxin transport and hence is upstream of ba1.
Expression analysis shows that bif2 levels are somewhat reduced in Bif1 mutants. Some of the reduction in bif2 expression could be explained by the absence of structures that express bif2, or this result could imply that Bif1 acts upstream of bif2. However, the synergistic effect observed in Bif1; bif2 double mutants implies that bif1 and bif2 have overlapping functions. Both mutants have a very similar phenotype but the double mutant is much more severe than either single mutant, indicating that bif1 and bif2 may play redundant roles in vegetative and inflorescence development. The dosage effect of the Bif1; bif2 interaction further supports that they affect the same process. From the results of the double-mutant and expression analyses, together with previous results, we propose that bif1 and bif2 both act upstream of auxin transport.
To determine the molecular mechanism by which bif1 regulates auxin transport, future work will identify the bif1 gene by map-based cloning. With the sequencing of the maize genome and the availability of the genome sequence of related grasses, chromosome walking is now routine in maize (Salvi et al. 2002; Wang et al. 2005; Alleman et al. 2006; Bortiri et al. 2006a,b; Satoh-Nagasawa et al. 2006; Taramino et al. 2007). Many regulators of auxin transport have been identified in other species; however, only pin and pid mutants have a pin inflorescence phenotype (Brown et al. 2001; Gil et al. 2001; Noh et al. 2001; Geisler et al. 2003; Geldner et al. 2003; Multani et al. 2003; Bennett et al. 2006; Sieburth et al. 2006). The closest maize homologs of pin and pid do not map to Bif1, indicating that the bif1 gene possibly may be a novel regulator of auxin transport in the inflorescence.
Acknowledgments
P.M. thanks Sarah Hake for her mentorship early in the development of the barren inflorescence project. We thank Tony Omeis, W. Scott Harkcom, and Bob Oberheim for plant care in the Department of Biology greenhouse and in the Departments of Horticulture and Crop and Soil Sciences farm sites in Rock Springs, Pennsylvania. We thank Carrie Barrios for performing some of the genetic crosses, Molly Saweikis for initial SEM analysis, Missy Hazen for training on the SEM, Deb Grove for assistance with real-time RT–PCR experiments, and undergraduate students Jason Hoar, Jeffrey Buterbaugh, Kim Phillips, Matt Davis, and Chris Cook for assistance with analysis of double mutants in the field. We thank members of the Braun and McSteen labs for discussion and critical reading of the manuscript. This research was funded by the Huck Institutes of Life Science graduate fellowship to S.B. and by National Science Foundation grant no. IOS-0416616 to P.M.
References
- Alleman, M., L. Sidorenko, K. McGinnis, V. Seshadri, J. E. Dorweiler et al., 2006. An RNA-dependent RNA polymerase is required for paramutation in maize. Nature 442 295–298. [DOI] [PubMed] [Google Scholar]
- Benjamins, R., A. Quint, D. Weijers, P. Hooykaas and R. Offringa, 2001. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development 128 4057–4067. [DOI] [PubMed] [Google Scholar]
- Benkova, E., M. Michniewicz, M. Sauer, T. Teichmann, D. Seifertova et al., 2003. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602. [DOI] [PubMed] [Google Scholar]
- Bennett, S. R. M., J. Alvarez, G. Bossinger and D. R. Smyth, 1995. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 8 505–520. [Google Scholar]
- Bennett, T., T. Sieberer, B. Willett, J. Booker, C. Luschnig et al., 2006. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 16 553–563. [DOI] [PubMed] [Google Scholar]
- Bommert, P., N. Satoh-Nagasawa, D. Jackson and H. Y. Hirano, 2005. Genetics and evolution of inflorescence and flower development in grasses. Plant Cell Physiol. 46 69–78. [DOI] [PubMed] [Google Scholar]
- Bortiri, E., and S. Hake, 2007. Flowering and determinacy in maize. J. Exp. Bot. 58 909–916. [DOI] [PubMed] [Google Scholar]
- Bortiri, E., G. Chuck, E. Vollbrecht, T. Rocheford, R. Martienssen et al., 2006. a ramosa2 encodes a LATERAL ORGAN BOUNDARY domain protein that determines the fate of stem cells in branch meristems of maize. Plant Cell 18 574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortiri, E., D. Jackson and S. Hake, 2006. b Advances in maize genomics: the emergence of positional cloning. Curr. Opin. Plant Biol. 9 164–171. [DOI] [PubMed] [Google Scholar]
- Brown, D. E., A. M. Rashotte, A. S. Murphy, J. Normanly, B. W. Tague et al., 2001. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 126 524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., and S. L. Dellaporta, 1994. Urea based plant DNA miniprep, pp. 526–527 in The Maize Handbook, edited by M. Freeling and V. Walbot. Springer-Verlag, New York.
- Cheng, P. C., R. I. Greyson and D. B. Walden, 1983. Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am. J. Bot. 70 450–462. [Google Scholar]
- Cheng, Y., X. Dai and Y. Zhao, 2007. Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19 2430–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. F., and Y. D. Zhao, 2007. A role for auxin in flower development. J. Integr. Plant Biol. 49 99–104. [Google Scholar]
- Cheng, Y. F., X. H. Dai and Y. D. Zhao, 2006. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 20 1790–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, S. K., N. Dagenais, J. Chory and D. Weigel, 2000. Regulation of auxin response by the protein kinase PINOID. Cell 100 469–478. [DOI] [PubMed] [Google Scholar]
- Clifford, H. T., 1987. Spikelet and floral morphology, pp. 21–30 in Grass Systematics and Evolution, edited by T. R. Soderstrom, K. W. Hilu, C. S. Campbell and M. E. Barkworth. Smithsonion Institution Press, Washington, DC.
- Coe, E. H., M. G. Neuffer and D. A. Hoisington, 1988. The genetics of corn, pp. 81–258 in Corn and Corn Improvement, edited by G. F. Sprague and J. W. Dudley. ASA-CSSA-SSSA, Madison, WI.
- Doebley, J., A. Stec and L. Hubbard, 1997. The evolution of apical dominance in maize. Nature 386 485–488. [DOI] [PubMed] [Google Scholar]
- Gallavotti, A., Q. Zhao, J. Kyozuka, R. B. Meeley, M. Ritter et al., 2004. The role of barren stalk1 in the architecture of maize. Nature 432 630–635. [DOI] [PubMed] [Google Scholar]
- Galweiler, L., C. H. Guan, A. Muller, E. Wisman, K. Mendgen et al., 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282 2226–2230. [DOI] [PubMed] [Google Scholar]
- Geisler, M., H. U. Kolukisaoglu, R. Bouchard, K. Billion, J. Berger et al., 2003. TWISTED DWARF1, a unique plasma membrane-anchored immunophilin-like protein, interacts with Arabidopsis multidrug resistance-like transporters AtPGP1 and AtPGP19. Mol. Biol. Cell 14 4238–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geldner, N., N. Anders, H. Wolters, J. Keicher, W. Kornberger et al., 2003. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112 219–230. [DOI] [PubMed] [Google Scholar]
- Gernart, W., 1912. A new subspecies of Zea mays L. Am. Nat. 46 616–622. [Google Scholar]
- Gil, P., E. Dewey, J. Friml, Y. Zhao, K. C. Snowden et al., 2001. BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 15 1985–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke, C. S., W. Ckurshumova, D. P. Vidaurre, S. A. Singh, G. Stamatiou et al., 2004. Overlapping and non-redundant functions of the Arabidopsis auxin response factors MONOPTEROS and NONPHOTOTROPIC HYPOCOTYL 4. Development 131 1089–1100. [DOI] [PubMed] [Google Scholar]
- Heisler, M. G., C. Ohno, P. Das, P. Sieber, G. V. Reddy et al., 2005. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15 1899–1911. [DOI] [PubMed] [Google Scholar]
- Hubbard, L., P. McSteen, J. Doebley and S. Hake, 2002. Expression patterns and mutant phenotype of teosinte branched1 correlate with growth suppression in maize and teosinte. Genetics 162 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish, E. E., 1996. Regulation of sex determination in maize. BioEssays 18 363–369. [Google Scholar]
- Irish, E. E., 1997. Class II tassel seed mutations provide evidence for multiple types of inflorescence meristems in maize (Poaceae). Am. J. Bot. 84 1502–1515. [PubMed] [Google Scholar]
- Jackson, D., B. Veit and S. Hake, 1994. Expression of maize knotted1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development 120 405–413. [Google Scholar]
- Kellogg, E. A., 2000. The grasses: a case study in macroevolution. Annu. Rev. Ecol. Syst. 31 217–238. [Google Scholar]
- Kellogg, E. A., 2007. Floral displays: genetic control of grass inflorescences. Curr. Opin. Plant Biol. 10 26–31. [DOI] [PubMed] [Google Scholar]
- Kerstetter, R. A., D. Laudencia-Chingcuanco, L. G. Smith and S. Hake, 1997. Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development 124 3045–3054. [DOI] [PubMed] [Google Scholar]
- Lee, S. H., and H. T. Cho, 2006. PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18 1604–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Livak, K. J., and T. D. Schmittgen, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the
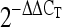 method. Methods 25 402–408. [DOI] [PubMed] [Google Scholar]
method. Methods 25 402–408. [DOI] [PubMed] [Google Scholar] - McSteen, P., 2006. Branching out: the ramosa pathway and the evolution of grass inflorescence morphology. Plant Cell 18 518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSteen, P., and S. Hake, 1998. Genetic control of plant development. Curr. Opin. Biotechnol. 9 189–195. [Google Scholar]
- McSteen, P., and S. Hake, 2001. barren inflorescence2 regulates axillary meristem development in the maize inflorescence. Development 128 2881–2891. [DOI] [PubMed] [Google Scholar]
- McSteen, P., and O. Leyser, 2005. Shoot branching. Annu. Rev. Plant Biol. 56 353–374. [DOI] [PubMed] [Google Scholar]
- McSteen, P., D. Laudencia-Chingcuanco and J. Colasanti, 2000. A floret by any other name: control of meristem identity in maize. Trends Plant Sci. 5 61–66. [DOI] [PubMed] [Google Scholar]
- McSteen, P., S. Malcomber, A. Skirpan, C. Lunde, X. Wu et al., 2007. barren inflorescence2 encodes a co-ortholog of the PINOID serine/threonine kinase and is required for organogenesis during inflorescence and vegetative development in maize. Plant Physiol. 144 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multani, D. S., S. P. Briggs, M. A. Chamberlin, J. J. Blakeslee, A. S. Murphy et al., 2003. Loss of an MDR transporter in compact stalks of maize br2 and sorghum dw3 mutants. Science 302 81–84. [DOI] [PubMed] [Google Scholar]
- Neuffer, M. G., and K. A. Sheridan, 1977. Dominant mutants from EMS treated pollen. Maize Newsl. 51 59–60. [Google Scholar]
- Neuffer, M. G., E. H. Coe and S. R. Wessler, 1997. The Mutants of Maize. Cold Spring Harbor Laboratory Press, Plainview, NY.
- Noh, B., A. S. Murphy and E. P. Spalding, 2001. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13 2441–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, K., J. Ueda, M. K. Komaki, C. J. Bell and Y. Shimura, 1991. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przemeck, G. K. H., J. Mattsson, C. S. Hardtke, Z. R. Sung and T. Berleth, 1996. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta 200 229–237. [DOI] [PubMed] [Google Scholar]
- Reinhardt, D., T. Mandel and C. Kuhlemeier, 2000. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 12 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt, D., E. R. Pesce, P. Stieger, T. Mandel, K. Baltensperger et al., 2003. Regulation of phyllotaxis by polar auxin transport. Nature 426 255–260. [DOI] [PubMed] [Google Scholar]
- Ritter, M. K., C. M. Padilla and R. J. Schmidt, 2002. The maize mutant barren stalk1 is defective in axillary meristem development. Am. J. Bot. 89 203–210. [DOI] [PubMed] [Google Scholar]
- Salvi, S., R. Tuberosa, E. Chiapparino, M. Maccaferri, S. Veillet et al., 2002. Toward positional cloning of Vgt1, a QTL controlling the transition from the vegetative to the reproductive phase in maize. Plant Mol. Biol. 48 601–613. [DOI] [PubMed] [Google Scholar]
- Satoh-Nagasawa, N., N. Nagasawa, S. Malcomber, H. Sakai and D. Jackson, 2006. A trehalose metabolic enzyme controls inflorescence architecture in maize. Nature 441 227–230. [DOI] [PubMed] [Google Scholar]
- Scanlon, M. J., 2003. The polar auxin transport inhibitor N-1-naphthylphthalamic acid disrupts leaf initiation, KNOX protein regulation, and formation of leaf margins in maize. Plant Physiol. 133 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan, W. F., 1988. Maize developmental genetics: genes of morphogenesis. Annu. Rev. Genet. 22 353–385. [DOI] [PubMed] [Google Scholar]
- Sieburth, L. E., G. K. Muday, E. J. King, G. Benton, S. Kim et al., 2006. SCARFACE encodes an ARF-GAP that is required for normal auxin efflux and vein patterning in Arabidopsis. Plant Cell 18 1396–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves, T., and I. Sussex, 1989. Patterns in Plant Development. Cambridge University Press, Cambridge, UK.
- Taramino, G., M. Sauer, J. L. Stauffer, D. Multani, X. Niu et al., 2007. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 50 649–659. [DOI] [PubMed] [Google Scholar]
- Veit, B., 2006. Stem cell signalling networks in plants. Plant Mol. Biol. 60 793–810. [DOI] [PubMed] [Google Scholar]
- Veit, B., R. J. Schmidt, S. Hake and M. F. Yanofsky, 1993. Maize floral development—new genes and old mutants. Plant Cell 5 1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernoux, T., J. Kronenberger, O. Grandjean, P. Laufs and J. Traas, 2000. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development 127 5157–5165. [DOI] [PubMed] [Google Scholar]
- Vollbrecht, E., L. Reiser and S. Hake, 2000. Shoot meristem size is dependent on inbred background and presence of the maize homeobox gene, knotted1. Development 127 3161–3172. [DOI] [PubMed] [Google Scholar]
- Vollbrecht, E., P. S. Springer, L. Goh, E. S. Buckler, IV and R. Martienssen, 2005. Architecture of floral branch systems in maize and related grasses. Nature 436 1119–1126. [DOI] [PubMed] [Google Scholar]
- Wang, H., T. Nussbaum-Wagler, B. Li, Q. Zhao, Y. Vigouroux et al., 2005. The origin of the naked grains of maize. Nature 436 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., and P. McSteen, 2007. The role of auxin transport during inflorescence development in maize, Zea mays (Poaceae). Am. J. Bot. 11 1745–1755. [DOI] [PubMed] [Google Scholar]